9 Intestine
9.1 Preamble
Intestinal transplantation remains challenging and controversial because of the complexity of the intestinal failure patient, the effectiveness of parenteral nutrition (PN), and the risks associated with transplanting the intestine.
The gut is a highly complex, highly immunogenic organ, and is exposed to the external environment of chemicals, parasites, viruses and bacteria. There is a poorly understood symbiotic relationship between the gut and the intestinal flora, which encompass many trillion bacteria. The gut ‘microbiota’ and the intestinal immune system have a complex relationship that includes tolerance to the native flora. It is therefore not surprising that, following transplantation, the intestine is prone to rejection, loss of the mucosal barrier, and subsequent systemic infection.
Several medical advancements preceded and permitted the development of intestinal transplantation in patients who have intestinal failure. The introduction of PN in the 1970s was followed by the development of intestinal rehabilitation and subsequent remedial intestinal surgery. The concept of a specialised service to manage intestinal failure and rehabilitation is more recent.11 Abu-Elmagd K. The concept of gut rehabilitation and the future of visceral transplantation. Nat Rev Gastroenterol Hepatol, 2015; 12(2): 108-20. ×
While the role of intestinal transplantation in the complex management of intestinal failure is still evolving, PN remains the primary therapy for both adults and children with intestinal failure.
It is estimated that approximately 200-250 patients in Australia and New Zealand are currently PN dependant, corresponding to a prevalence of 8-10 per million population (personal communication Baxter Healthcare 2015). This is consistent with prevalence estimates from Europe, which range from 3-12 per million population; by contrast, prevalence of PN dependency in the United States is estimated at 30-40 per million population. 2 Most patients on PN are stable, and consideration for transplantation is currently limited to those who have no chance of intestinal recovery and have potential life-threatening PN-related complications.2 Pironi L, Goulet O, Buchman A, et al. Outcome on home parenteral nutrition for benign intestinal failure: a review of the literature and benchmarking with the European prospective survey of ESPEN. Clin Nutr, 2012; 31(6): 831-45. ×
As short-term patient and graft survival have increased, attention has turned to improving the long-term outcomes of intestinal transplantation. Long-term survival must be factored into any decision to transplant an individual patient where survival on PN may approximate or exceed that of intestinal transplantation. The improved outcomes of intestinal transplantation and the potential for long-term survival raises the possibility of considering intestinal transplantation for stable patients who have a poor quality of life or — in high-risk patients — before the development of life-threatening PN-related complications.
Management of intestinal failure patients in dedicated centres with multidisciplinary teams has been associated with improved survival and fewer complications. 3-5 Given the small number of patients who might be considered intestinal transplant candidates in Australia and New Zealand, and the fact that they are scattered over a large area, it has been recommended that there should be a single intestinal transplant programme supported by organised intestinal rehabilitation programmes across the two countries. 63 Avitzur Y, Wang JY, de Silva NT, et al. Impact of Intestinal Rehabilitation Program and Its Innovative Therapies on the Outcome of Intestinal Transplant Candidates. J Pediatr Gastroenterol Nutr, 2015; 61(1): 18-23. 4 Stanger JD, Oliveira C, Balckmore C, et al. The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: a systematic review and meta-analysis. J Pediatr Surg, 2013; 48(5): 983-92. 5 Mazariegos GV. Rehabilitation of intestinal failure: new paradigms for medical, surgical and transplant therapy. Curr Opin Organ Transplant, 2010; 15(3): 322-3. ×6 Intestinal Failure in Australian and New Zealand: Current services, gap analysis and service planning guidelines. DLA Piper for Health Policy Advisory Committee on Technology, February 2014. ×
Types of intestinal transplantation
Intestinal transplantation incorporates several transplant procedures, and can range in complexity from an isolated intestinal graft to replacement of the entire abdominal cavity including stomach, duodenum, pancreas, small intestine, liver and possibly colon. Kidney transplantation may also be contemplated.
The decision for an individual patient as to which organs to replace can be difficult. Intestinal failure associated liver disease (IFALD) is common and liver function is important in determining whether the liver should also be replaced. Advanced fibrosis, cirrhosis or severe cholestasis and the presence of portal hypertension mandate the liver should be included in the transplantation procedure.
In surgical practice, the graft options centre around isolated intestinal replacement versus the need to include the liver. A multi-visceral graft includes the liver, stomach, duodenum, pancreas and small intestine, whereas a modified multi-visceral graft does not include the liver.7-9 The multi-visceral graft can also include the spleen and colon. The factors that determine the choice of graft ‘cluster’ include the aetiology of the intestinal failure and the functional state of the liver and gastric motility. The type of graft is tailored to the individual patient. Organs that are functioning will not be replaced.7 Abu-Elmagd KM. The small bowel contained allografts: existing and proposed nomenclature. Am J Transplant, 2011; 11(1): 184-5. 8 Abu-Elmagd KM. Preservation of the native spleen, duodenum, and pancreas in patients with multivisceral transplantation: nomenclature, dispute of origin, and proof of premise. Transplantation, 2007; 84(9): 1208-9. 9 Cruz RJ Jr, Costa G, Bond G, et al. Modified “liver-sparing” multivisceral transplant with preserved native spleen, pancreas, and duodenum: technique and long-term outcome. J Gastrointest Surg, 2010; 14(11): 1709-21. ×
9.2 Parenteral Nutrition
There is a medical preference for enteric feeding, if at all possible, because of the reduced risk of systemic infection, venous thrombosis and liver dysfunction in comparison with PN. However PN remains the nutritional mainstay for patients who cannot eat or whose gastrointestinal tract cannot support enteral nutrition sufficient to meet the metabolic demands of the patient. The great majority of patients will have short-term surgical or medical conditions with no intention that PN will be used long-term, and an intestine that will allow them to return to full enteral feeding once their condition is resolved.
In patients with irreversible intestinal failure PN remains the gold standard for treatment. The five- and ten-year survival for children receiving total parenteral nutrition (TPN) is 89% and 81% respectively; the five- and ten-year survival for adults receiving TPN is 70% and 55% respectively. 2,102 Pironi L, Goulet O, Buchman A, et al. Outcome on home parenteral nutrition for benign intestinal failure: a review of the literature and benchmarking with the European prospective survey of ESPEN. Clin Nutr, 2012; 31(6): 831-45. 10 Colomb V, Dabbas-Tyan M, Taupin P, et al. Long-term outcome of children receiving home parenteral nutrition: a 20-year single-center experience in 302 patients. J Pediatr Gastroenterol Nutr, 2007; 44(3): 347-53. ×
However, long-term PN can result in life-threatening complications. Intestinal transplantation has usually been reserved for patients who develop the following problems:11-1311 Garg M, Jones RM, Vaughan RB, Testro AG. Intestinal transplantation: current status and future directions. J Gastroenterol Hepatol, 2011. 26(8): 1221-8. 12 Fishbein TM. Intestinal transplantation. N Engl J Med, 2009; 361(10): 998-1008. 13 Dibb M, Teubner A, Theis V, et al. Review article: the management of long-term parenteral nutrition. Aliment Pharmacol Ther, 2013; 37(6): 587-603. ×
The development of IFALD—this can occur in up to half of all TPN patients and is associated with a dramatic reduction in patient survival.13 IFALD can result in advanced fibrosis or cirrhosis or severe cholestasis. Portal hypertension may develop, manifested by splenomegaly, thrombocytopenia, gastro-oesophageal varices or stomal bleeding. Liver biochemistry often is a poor indicator of the extent of liver injury, so liver biopsy and/ or non-invasive assessment of liver fibrosis are important considerations in the longer-term management of these patients.13 Dibb M, Teubner A, Theis V, et al. Review article: the management of long-term parenteral nutrition. Aliment Pharmacol Ther, 2013; 37(6): 587-603. ×
Central line access failure—as evidenced by central venous thrombosis of two or more central veins, pulmonary embolism, superior vena cava syndrome or chronic venous insufficiency.
Severe sepsis—usually secondary to catheter-related blood stream infections that require hospitalisation, or a single episode of line-related fungemia, septic shock or acute respiratory distress syndrome.
Severe dehydration—frequent episodes of severe dehydration despite intravenous fluid supplementation in addition to TPN.
PN in Australia and New Zealand
PN is widely available in Australia and New Zealand. However, there is little coordination and currently no operating central registry or national audit of PN patients.
A distinction should be made between hospitals able to offer PN and those that have a formal intestinal failure/ intestinal rehabilitation service. There is a dedicated paediatric intestinal failure service at the Royal Children’s Hospital in Melbourne and the Starship Children’s Hospital in Auckland, but no other recognised intestinal failure service. Intestinal failure, particularly in adults, is treated ad hoc, largely due to the low incidence of gastrointestinal tract pathology and the wide geographic distribution of the few affected patients.
9.3 Intestinal transplantation in Australia
Intestinal transplantation is an emerging therapy in Australia and New Zealand. The low prevalence of intestinal failure across the two countries suggests a need for a single bi-national adult and paediatric transplant centre, as transplantation may be indicated in only four or five patients per year across Australia and New Zealand.
An intestinal transplantation programme has been recently established at the Austin Hospital and Royal Children’s Hospital in Melbourne. 11 The first intestinal transplant (liver-intestine) was performed in 2012. 14 A total of three patients have been transplanted (one adult, two children), and there is currently an active waiting list of children and adults.11 Garg M, Jones RM, Vaughan RB, Testro AG. Intestinal transplantation: current status and future directions. J Gastroenterol Hepatol, 2011. 26(8): 1221-8. ×14 Garg M, Jones RM, Mirza D, et al. Australia’s first liver-intestinal transplant. Med J Aust, 2012; 197(8): 463-5. ×
The intestinal transplantation programme is not currently funded, therefore the funding for transplantation of an individual patient is negotiated on an ad hoc basis with each referring state and New Zealand. This often adds considerably to the time taken to assess the patient, complete their work-up, and activate them on the waiting list.
A single bi-national intestinal transplantation service would ideally be supported by a limited network of intestinal rehabilitation centres that would act as a referral base for intestinal failure patients. Management of patients post-transplantation would likely be done at an existing liver transplant centre with expertise in the management of immunosuppression.
9.4 Recipient eligibility
Intestinal failure occurs when intestinal absorption of fluid and nutrients becomes inadequate and life can only be sustained by the use of intravenous PN and fluids. The access line and long-term access can become life-threatening issues, particularly due to the risk of infection and large vein thrombosis.
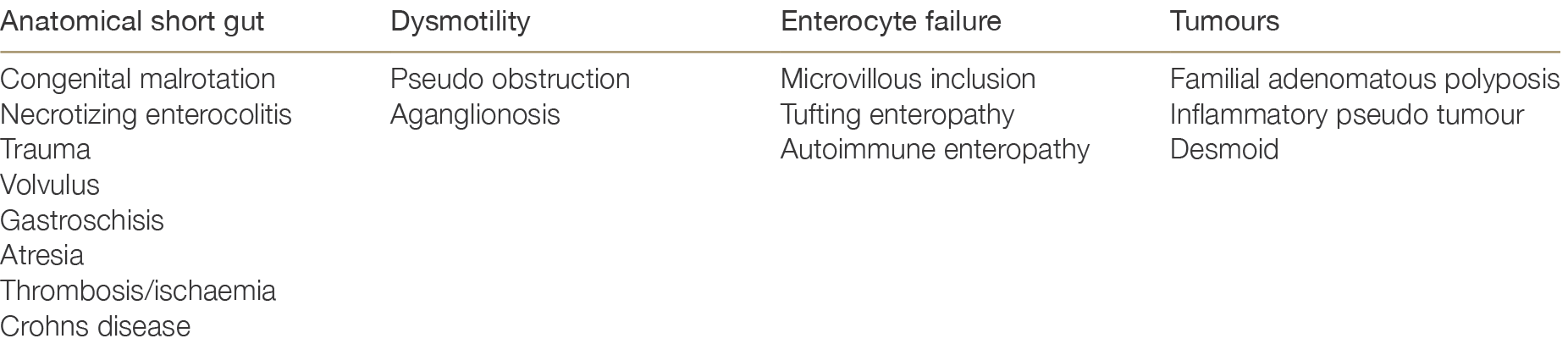
Approximately 70% of intestinal failure in both adults and children is due to anatomical short gut. However there are multiple other causes, which are summarised in Table 9.1.
Table 9.1: Causes of intestinal failure
Quality of life is impaired to some degree for many patients with intestinal failure, and to a severe degree for a minority. Survival requires daily intervention. This burden is compounded by the inability to eat and the enormous social dysfunction that this entails. Hospitalisation can become frequent and costly, both financially and psychologically. Patient survival is precarious, and the social impairment and psychopathology of a severe chronic disease are common.
The following anatomical combinations can be associated with full enteral recovery, and hence aggressive attempts at intestinal rehabilitation should be undertaken before intestinal transplantation is considered:1515 Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology, 1999; 117(5): 1043-50. ×
Residual small intestine of >100 cm with a stoma (no colon in continuity)
Small intestine >60 cm with jejunocolonic anastomosis (part of the colon in continuity)
Small intestine >30 cm, including the ileum and ICV, in continuity with the entire colon.
Of the initial 52 patients referred to Melbourne for consideration for intestinal transplantation, more than 60 % were entirely dependent on PN, with the remaining 40% being treated with a combination of PN and enteral/oral nutrition or enteral/oral nutrition plus intra-venous fluids.
Patients with the following are more likely to remain dependent on PN and hence may ultimately become candidates for transplantation:
Gut length—very short jejunum, no ileum, no ileocecal valve (ICV), no colon
Mucosal disease
Motility disorders
Abdominal wall defects
Radiation enteritis
Age—children may do worse on PN
High-grade intestinal obstruction
Long duration of PN feeding (>2 years)
A post-absorptive plasma citrulline level <20 µmol/L (half of normal adult value).
9.4.1 Inclusion criteria
It is important to realise that only a small proportion of patients with intestinal failure on PN will be referred for transplantation and subsequently accepted and transplanted. Intestinal transplantation is a recognised treatment for patients with intestinal failure, but will usually not be considered as an option for stable patients who are coping well with PN. Instead, intestinal transplantation is currently considered only for patients with known irreversible intestinal failure who have life threatening complications of PN or fluid management or have significant limitations to their quality of life that have become life-threatening. This so-called “PN failure” has been defined in the USA as one or more of the following:1212 Fishbein TM. Intestinal transplantation. N Engl J Med, 2009; 361(10): 998-1008. ×
Impending or overt liver failure due to IFALD
Thrombosis of two or more central veins
Two or more episodes per year of catheter-related blood stream infections
A single episode of line-related fungemia, septic shock or acute respiratory distress syndrome
Frequent episodes of severe dehydration despite intravenous supplementation in addition to PN.
In addition to the criteria above, there is a small group of patients who have aggressive, locally destructive abdominal desmoid tumours, who may be eligible for intestinal transplantation in the absence of PN failure. 1616 Cruz RJ Jr, Costa G, Bond GJ, et al. Modified multivisceral transplantation with spleen-preserving pancreaticoduodenectomy for patients with familial adenomatous polyposis “Gardner’s Syndrome”. Transplantation, 2011; 91(12):1417-23. ×
There is debate about who should remain on long-term PN and regarding the ability to predict success of intestinal rehabilitation in an individual patient. The ability to predict rehabilitation success or failure may facilitate early consideration of intestinal transplantation before the onset of life-threatening complications.
9.4.2 Exclusion criteria
Exclusion criteria overlap with those listed for liver transplantation (see Chapter 6).
In summary, contraindications to intestinal transplantation include:1717 Steinman TI, Becker BN, Frost AE, et al. Guidelines for the referral and management of patients eligible for solid organ transplantation. Transpl, 2001; 71(9): 1189–204. ×
Potential for intestinal recovery
Severe wasting and cachexia
Drug dependence considered likely to impair survival
Primary or metastatic cancer (with exception of desmoid tumours)
Ongoing or recurrent infections that are not responding to treatment
Significant cardiac or pulmonary pathology
Demonstrated patient non-compliance or significant psychiatric or social risk
Potential complications from immunosuppressive therapy that are unacceptable to the patient
Total loss of central line access.
9.4.3 Referral for intestinal transplantation
At any instant, 10 – 25% of adults and children on long-term PN may have one of the complications listed in Section 9.4.1 that are an indication for intestinal transplantation. It is therefore estimated that fewer than 10 patients per year in Australia and New Zealand would be considered for intestinal transplantation.
However, determining whether a patient should be transplanted is often difficult. Early referral is preferred as it allows sufficient time to assess the patient, modify treatment and consider the need for transplantation.
In patients for whom loss of central venous access is an indication, referral should be made prior to the patient losing all access as central venous access is necessary to survive the transplant operation, as well as for adequate postoperative care.
Over 70% of Australian patients referred to Melbourne had at least one life-threatening complication of PN at presentation. Five patients (10%) exhibited three life-threatening complications of PN: liver failure, impending loss of venous access and recurrent line sepsis; 11 patients (21%) displayed two complications and 20 patients (38%) presented with one complication.
Outcome of referral of patients with intestinal failure to 2018
Ninety-four patients have been referred to Melbourne since the intestinal transplantation service was established in 2010, including 65 adults (mean age 40 years) and 29 children (mean age 6 years). Sixty-seven percent have been either deferred or rejected from wait-listing for various reasons (75% with either ‘stable’ disease or not meeting transplant criteria; 16% too unwell for transplant; 9% unsuitable for psychosocial reasons).
Seven patients (7%) have so far died prior to transplantation, while awaiting transplantation or during the assessment period. Causes of death included sepsis and intracranial bleed.
Seven patients (four adults, three children) have undergone intestinal multivisceral transplantation (in all but one cases combined with liver transplantation). All achieved enteral autonomy. Patients are eight months to eight years post-transplantation. There has been one death due to respiratory failure with a functioning graft at three months post-transplant.
9.4.4 Assessment and acceptance
While there is no specific upper age limit for intestinal transplantation, most potential recipients are likely to be under 50 years of age. Patient adherence to medical treatment is critical to success. A stable social and psychological history is mandatory because of the intensity of the pre- and post-operative procedures and the ongoing medical risks.
Most patients will have undergone multiple abdominal operations that add to the operative risk. The abdominal cavity may be contracted and small with limited space in which to place a new graft.
A detailed assessment of the venous anatomy is mandatory. Thrombosis of the major vessels is common due to the prolonged intravenous access associated with PN. This may include complete thrombosis of the innominate or jugular veins, the superior vena cava and inferior vena cava. Vein mapping is essential to enable planning of the operation and anaesthetic access. In some patients who have lost major veins and where current intravenous access may be via direct atrial or lumbar caval lines, lack of access may preclude transplantation.
Co-morbidities are common in intestinal failure patients, and will influence the decision to proceed with transplantation. End-stage kidney disease is frequent, often due to long-term hydration issues and occasionally due to renal oxalosis as a complication of short bowel syndrome. In this case, combined kidney and intestinal transplantation may be considered.
Sensitisation and antibody status are critical to the success of intestinal transplantation, which will only be successful where there is a negative crossmatch between the recipient and the donor. Preformed HLA antibodies in the potential recipient make donor matching difficult and often impossible. Currently there are attempts to moderate donor specific antibodies (DSAs) in recipients with high titre and high panel reactive antibodies (PRA).
Assessment for intestinal transplantation may take many months, hence early referral is recommended. It usually takes this long to assess the patient and their response to various therapies, including surgery, in the hope that intestinal transplantation can be avoided.
9.4.5 Retransplantation
Re-transplantation is possible, but has a high failure rate when compared to primary transplantation.18 This is largely due to immunologic factors, which make rejection of the second transplant more likely, the presence of sepsis associated with failure of the primary graft, and other organ system failures. Liver-inclusive intestinal retransplantation offers a better long-term outcome when compared to liver-free retransplantation. 1918 Desai CS, Khan KM, Gruessner AC, et al. Intestinal retransplantation: analysis of Organ Procurement and Transplantation Network database. Transplantation, 2012; 93(1): 120-5. ×19 Wu G and Cruz RJ. Liver inclusion improves outcomes of intestinal retransplantation in adults. Transplantation, 2015; 99(6): 1265-72. ×
9.5 Donor assessment
The selection of appropriate deceased donors is critical to success of intestinal transplantation. In general, only stable donors who meet the criteria described below would be considered for intestinal transplantation. Most of the criteria for liver donor suitability also apply to intestinal donation (see Chapter 6).
The “ideal intestinal donor” is quite uncommon, hence interstate donors will be considered for all potential recipients. An ideal donor would be <50 years of age and donate via the DNDD pathway. Donors between 50 and 60 years of age will be considered if other factors are favourable.
Recipients must be ABO-compatible with the donor. Therefore, O universal donors can be considered for A, AB, or B recipients. The EBV and CMV status of the donor will also influence recipient selection because of the morbidity caused to naive recipients who develop a primary viral infection after transplantation.
In terms of technical factors affecting donor suitability, the gut is sensitive to ischaemia and hypotension therefore intestinal donors must have limited inotrope exposure, low volume or no blood transfusion and stable haemodynamics. The intestine does not tolerate cold storage and should be transplanted in the shortest possible time frame, ideally in under six hours. Irreversible intestinal damage has been observed after approximately five hours of cold ischemia. 20 Due to previous abdominal surgery, the recipient explant operation may take several hours and this will need to be factored into the timing of the donor retrieval operation.20 Cicalese L, Sileri P, Green M, et al. Bacterial translocation in clinical intestinal transplantation. Transplantation, 2001; 71(10): 1414-7. ×
The state of the donor liver will affect the decision to accept the intestine for transplantation. Further, the retrieving surgeon’s opinion of the intestine at the time of surgery and after perfusion is critical to the decision that the transplant should proceed.
Donors and recipients need to be size-matched because of the limited abdominal space. Donors should to be between 50% and 100% of recipient weight. Due to a lack of size-matched organs for paediatric recipients, reduced size intestine with or without liver transplantation has been performed elsewhere. It is not anticipated that this will occur at the Melbourne unit in the near future. Only DNDD paediatric donors are suitable for intestinal donation (see Chapter 11).
An isolated intestine can be retrieved as part of the retrieval of other abdominal organs. Intestinal donation will not interfere with simultaneous liver, whole organ pancreas or kidney retrieval.
9.5.1 Tissue typing and cross match
The gut is highly immunogenic and, like the kidney, is sensitive to the presence of circulating donor-specific HLA antibodies. It has become clear that donor-specific HLA antibodies (DSAs) are implicated in medium-long term intestinal allograft dysfunction and graft loss. 21,2221 Berger M, Zeevi A, Farmer DG, et al. Immunologic challenges in small bowel transplantation. Am J Transplant, 2012; 12 Suppl 4: S2-8. 22 Abu-Elmagd KM, Wu G, Costa G, et al. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant, 2012; 12(11): 3047-60. ×
Intestinal transplantation will only be performed upon the review of a virtual crossmatch. The difficulty in finding a suitable donor for a given recipient can be predicted during the work-up stage by the assessment of recipient DSAs. Recipients with multiple and high-level DSAs will have a high PRA and a high chance of an incompatible crossmatch with most donors. Additional comprehensive information is available within the National Histocompatibility Guidelines: https://tsanz.com.au/storage/Guidelines/TSANZ_ NationalHistocompatibilityAssessmentGuidelineForSolidOrganTransplantation_04.pdf
Although Luminex technology allows for ‘virtual’ crossmatching, a physical crossmatch can be requested for urgent listings where there has been insufficient time for antibody testing. This must be factored into the donation process and may delay organ retrieval, especially if the donor is in a regional hospital and the donor’s blood needs to be transported to the state tissue-typing laboratory.
9.6 Allocation
Competition between recipients is unlikely to be a problem during allocation because of the small number of patients on the intestinal transplant waiting list and the specific requirements of each recipient. ABO matching, DSA status, crossmatch results, size-matching and the availability of organs will usually point towards a single recipient.
There is currently no accepted method of ranking wait-listed patients in the context of intestinal transplantation. MELD score is not suitable for intestinal transplantation patients, who may have relatively mild liver disease.
If multiple patients are listed, they will be ranked on clinical criteria based on physician assessment. This will prioritise patients at greatest risk of dying on the waiting list death and take into account those likely to have the best post-transplant outcomes. If two recipients are otherwise both well matched, the treating physicians will allocate the donor organs to the recipient assessed to be in the greatest need (i.e. the sickest patient).
The prioritisation system has to assess the different risk factors for death, including liver failure, recurrent sepsis, fluid issues and loss of vascular access. International experience has demonstrated that patients who require a liver-intestine transplant have the highest waiting list mortality of all potential solid organ transplant recipients. 23 For this reason, in December 2012 the Liver and Intestinal Transplant Committee (LITAC) approved a new urgent list category (Category 2c) for all patients awaiting intestinal transplantation who also require liver transplantation. (See Section 6.3.3). Further, the Renal Transplant Advisory Committee (RTAC) has endorsed the allocation of a kidney (if required) to accompany the intestine (and other abdominal organs as necessary), including interstate donors. Individual patient approval will be obtained from RTAC given the infrequent need for this to occur.23 Fryer J, Pellar S, Ormond D, et al. Mortality in candidates waiting for combined liver-intestine transplants exceeds that for other candidates waiting for liver transplants. Liver Transpl, 2003; 9(7): 748-53. ×
The active intestinal/multivisceral transplantation waiting list for both adults and children is reviewed regularly and circulated weekly to all liver transplant units in Australia and New Zealand.
With time, it is anticipated that transplant activity will increase and allocation criteria may need to be reviewed accordingly.
9.7 Multi-visceral intestinal allocation versus liver-pancreas allocation
An isolated intestinal graft will not interfere with the retrieval and transplantation of other organs and can be retrieved concurrently. Patients who undergo a multivisceral intestinal transplant may need an organ that would otherwise be allocated to a patient on the liver, pancreas or kidney waiting list. There is no simple way of making this allocation decision between the potential recipients competing for the same organs. Allocation will take account of the competing needs of non-intestinal transplant candidates waiting for organs such as liver and pancreas that may be wanted for the intestinal recipient. A suitable multivisceral intestinal graft may be waived because there is a potential liver recipient who will die without urgent transplantation (e.g. a Category 1 listed liver recipient—see Table 6.2).