Appendix B
Process report
Background
The Organ and Tissue Authority (OTA) was established on 1 January 2009 with the aim of creating a nationally consistent and coordinated approach to organ and tissue donation for transplantation. Prior to the creation of OTA, the allocation of organs for transplantation was guided by state-specific guidelines, hospital protocols and protocols developed by the Transplantation Society of Australia and New Zealand (TSANZ) and the Australasian Donation and Transplant Coordinators Association (ADTCA).
On 16 January, 2009, as part of the Australian Government’s National Reform Agenda—A World’s Best Practice Approach to Organ and Tissue Donation for Transplantation – the Australian Government Department of Health and Ageing (subsequently transferred to the Organ and Tissue Authority) provided funding to TSANZ to enhance the role of its Advisory Committees and to convene a multidisciplinary working party of transplant clinicians, health-care professionals and consumer representatives to develop nationally uniform eligibility criteria and allocation protocols for deceased donor organ transplantation. The members of the original working party comprised a panel of transplantation clinicians in the specialty fields of cardiology, nephrology, respiratory medicine and surgery (Table B.1).
The initial draft of this document underwent a comprehensive public consultation process from August 2009 to April 2010. Version 1.1 of the TSANZ Organ Transplantation from Deceased Donors: Consensus Statement on Eligibility Criteria and Allocation Protocols (the Consensus Statement) was released by TSANZ in June 2011, and subsequent revisions were published in 2012, 2014 and 2015.
By 2015, in light of new scientific evidence and emerging technologies and practices, a full review of the Consensus Statement was deemed necessary. Concurrently, the National Health and Medical Research Council (NHMRC) commenced the development of Ethical Guidelines for Organ Donation and Transplantation (the Ethical Guidelines). The revisions to the Consensus Statement were conducted in parallel with the development of the Ethical Guidelines, and as a consequence were informed by the content of this document. The former Consensus Statement is now replaced by the Clinical Guidelines for Organ Transplantation from Deceased Donors (the Clinical Guidelines), with Version 1.0 of this document released in April 2016.
Table B1: Membership of the working party that developed the Consensus Statement on Eligibility Criteria and Allocation Protocols.

Development
The Clinical Guidelines are written in a way that makes them accessible to the wider community, however the primary target audience is health professionals within the donation and transplantation sectors. The Clinical Guidelines incorporate the latest national and international evidence and reflect current practice in Australia and New Zealand. Decisions with respect to the content and wording of each organ-specific chapter were made by the relevant TSANZ Advisory Committee, under the leadership of the respective Advisory Committee Chairs.
The following issues were declared outside the scope of the Clinical Guidelines:
The process of organ donation
Transplantation of human tissue
Transplantation of organs from living donors to a related (emotionally or biologically) recipient
Transplantation of gametes, ovarian or testicular tissue, or embryos for reproductive purposes
Xenotransplantation.
Targeted consultation on Version 1.0 of the Clinical Guidelines occurred between August 1 and September 15, 2015. Written submissions arising from the targeted consultation were then considered by the relevant TSANZ Advisory Committee and revisions made where appropriate. Submissions were not made publicly available.
Table B2: Contributors to the content development of the TSANZ Clinical Guidelines for Organ Transplantation from Deceased Donors (Version 1.0, 2016)
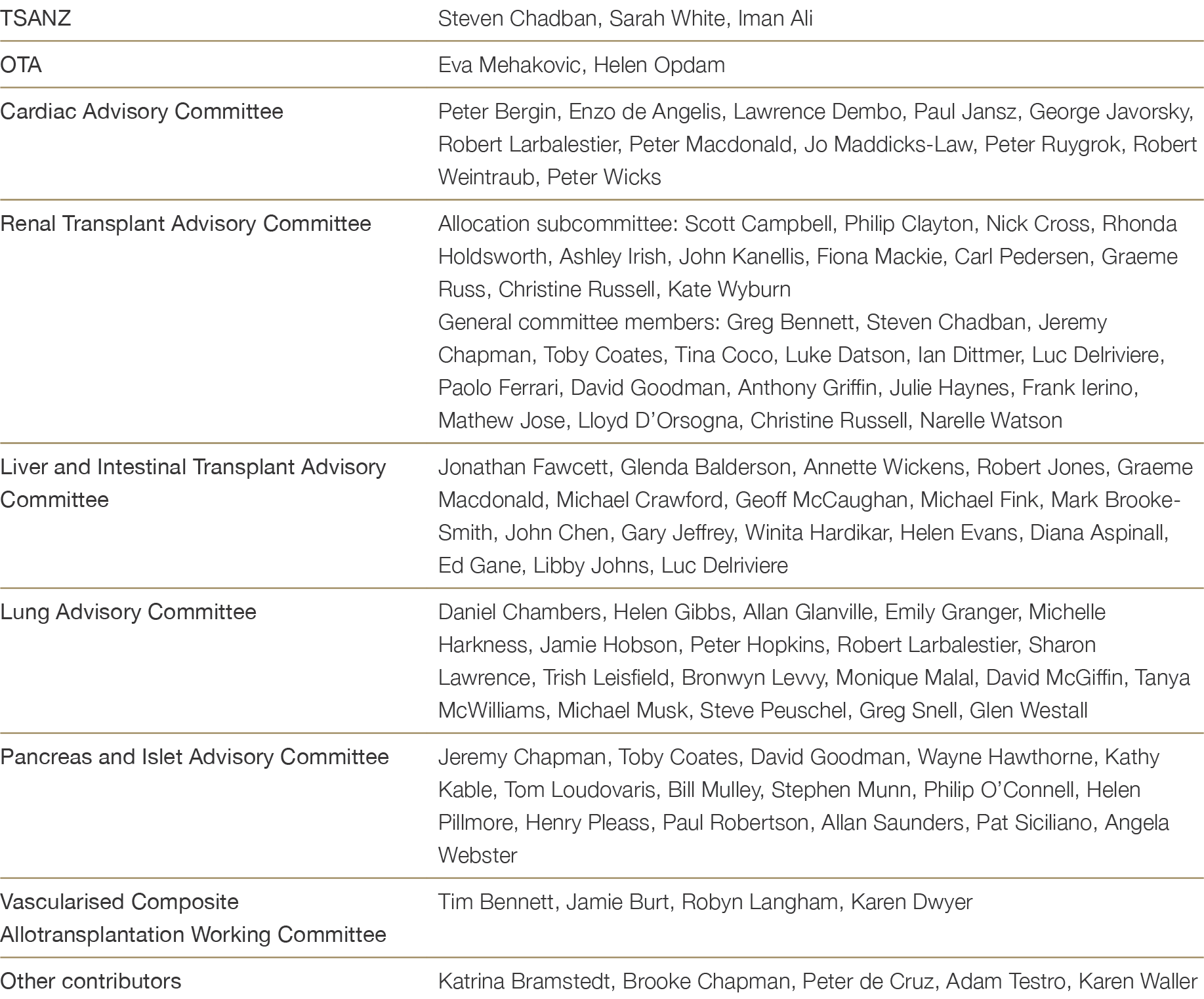
Table B3: List of organisations invited to submit comments on the draft Clinical Guidelines, 2nd September 2015 to 6th October 2015nd No reference text available.×th No reference text available.×

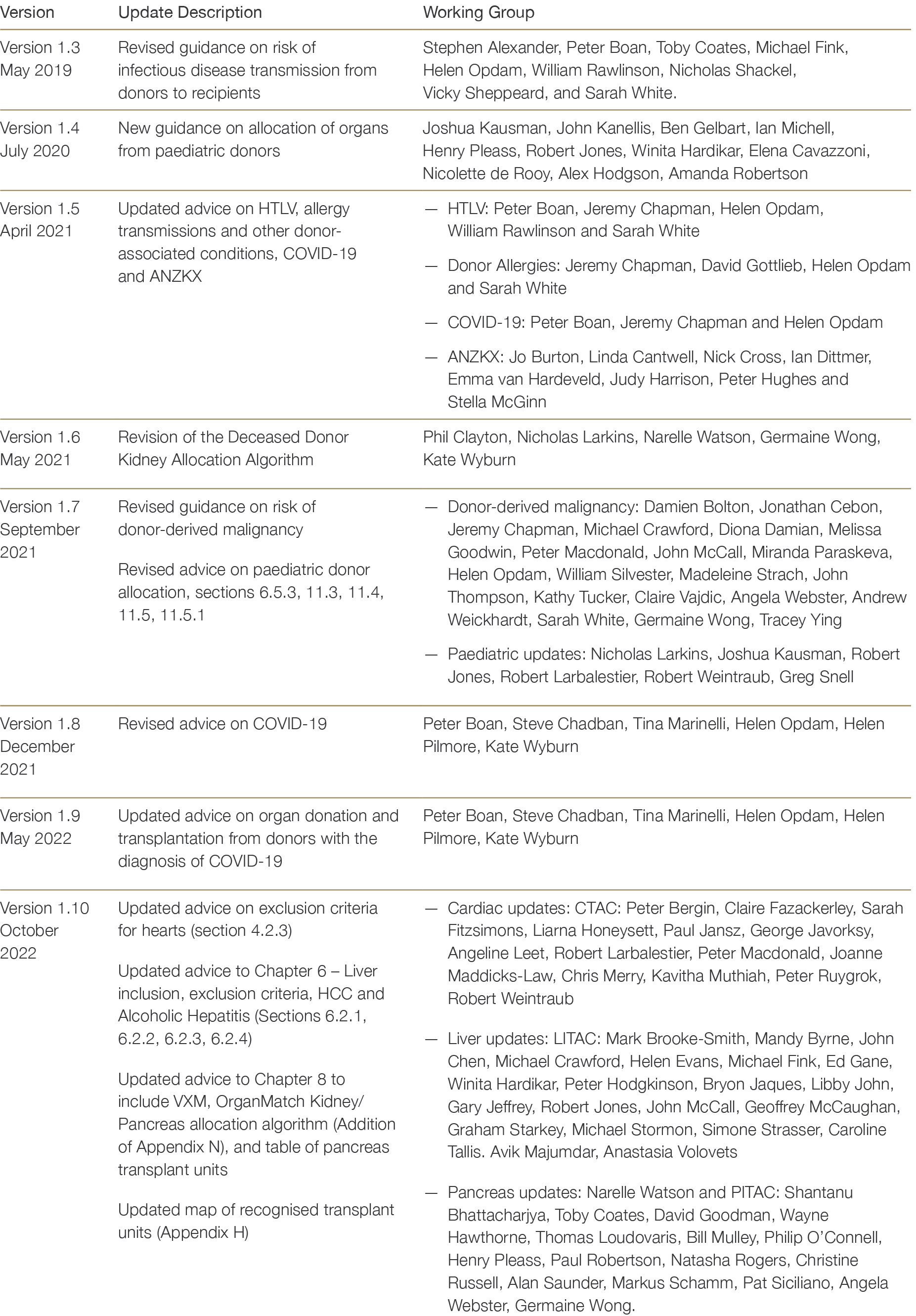
Version Updates
To maintain clinical relevance and community acceptability, eligibility criteria and allocation protocols must undergo periodic revision to account for evolving national and international evidence, clinical best practice, and trends in donor availability and acceptability criteria. The Clinical Guidelines are therefore regularly reviewed by the TSANZ Advisory Committees, with updates made on an ad hoc basis to reflect changes in clinical practice.
Updates to the Clinical Guidelines and their contributers are summarised below in Table B4.
Table B4: Contributors to updates to the TSANZ Clinical Guidelines for Organ Transplantation from Deceased Donors