2 Organ donor suitability
The majority of transplantation in Australia and New Zealand is possible because of deceased donation, including all heart, lung, pancreas, most liver, and approximately 70% of all kidney transplantation.1 Deceased donation is based on altruistic decisions of individuals and/or their families to donate organs to benefit other people. In Australia and New Zealand, as in all countries, there are more people who might benefit from organ transplantation than there are donor organs available. This is largely due to the small proportion of people who die in the specific circumstances under which organ donation is currently medically feasible (approximately 2% of hospital deaths). The framework within which deceased organ donation occurs includes the laws and regulations that govern the determination of death and the use of human organs and tissues for transplantation, as well as the policies and guidelines that direct clinical practice. 2,3,4,51 ANZDATA Registry. 45th Annual Report. Australian and New Zealand Dialysis and Transplant Registry, Adelaide, Australia, 2021. ×2 The Australian and New Zealand Intensive Care Society Statement on Death and Organ Donation. Melbourne. Edition 4.1 2021. 3 National Health and Medical Research Council (2025). Ethical guidelines for cell, tissue and organ donation and transplantation in Australia. Canberra: National Health and Medical Research Council 4 Best Practice Guideline for Donation after Circulatory Determination of Death (DCDD) in Australia Edition 1.0 October 2021, Australian Government Organ and Tissue Authority. 5 Report of the Law Reform Commission on Human Tissue Transplants. Australian Law Reform Commission, Australian Government Publishing Service, Canberra, Australia, 1977. ×
2.1 The organ donation process
2.1.1 Prerequisites for deceased organ donation
Before organ donation can take place:
The donor must have been declared deceased by qualified physicians using accepted guidelines that are consistent with the laws and regulations of the jurisdiction in which the donor has died (see ANZICS statement2), and2 The Australian and New Zealand Intensive Care Society Statement on Death and Organ Donation. Melbourne. Edition 4.1 2021. ×
Consent to organ donation must have been given and documented according to the laws and regulations of that jurisdiction.
It is the formal responsibility of a designated officer appointed by the hospital authorities, reinforced by the Donation Specialist Coordinator and all surgeons in charge of donor surgical teams, to confirm that these laws and regulations have been fully complied with and documented appropriately before proceeding to the retrieval of organs.
2.1.2 Determination of death and pathways to organ donation
Criteria for declaring death in Australia and New Zealand are: 2,52 The Australian and New Zealand Intensive Care Society Statement on Death and Organ Donation. Melbourne. Edition 4.1 2021. 5 Report of the Law Reform Commission on Human Tissue Transplants. Australian Law Reform Commission, Australian Government Publishing Service, Canberra, Australia, 1977. ×
Irreversible cessation of all function of the brain of the person, or
Irreversible cessation of the circulation of blood in the body of the person.
Death declared according to neurological criteria (brain death) is only possible when the person is maintained on a mechanical ventilator, usually whilst receiving treatment in an intensive care unit (ICU). Conditions causing sufficient brain injury to culminate in neurological death include haemorrhagic or occlusive stroke, trauma, hypoxic-ischaemic brain injury following a cardiac arrest, central nervous system infections and tumours. There are strict criteria and procedures for the determination of neurological death in Australia and New Zealand, which are outlined in the clinical guidelines of the Australian and New Zealand Intensive Care Society.2 Donation after neurological determination of death (DNDD) results in better transplant outcomes for some organs, and is more predictable with only a small proportion of cases not proceeding to the surgical retrieval of transplantable organs. DNDD is limited by the low and decreasing incidence of stroke, brain trauma and other causes of neurological death observed in many developed countries including Australia and New Zealand. This means that DNDD is possible in fewer than 1% of the deaths that occur in hospital.2 The Australian and New Zealand Intensive Care Society Statement on Death and Organ Donation. Melbourne. Edition 4.1 2021. ×
Death is more commonly determined using circulatory criteria and—in a limited number of such circumstances— organ donation may be possible. Donation after circulatory determination of death (DCDD) in Australia and New Zealand can occur after a decision has been made to withdraw treatment because it is considered no longer to be in the person’s best interest.4 This decision is usually reached by the healthcare staff and family, although in very rare and exceptional circumstances the decision may be made by the conscious, competent patient. The majority of patients suitable for DCDD are receiving mechanical ventilation and/or other cardio-respiratory supportive treatments in intensive care units. If loss of cardiac output with absence of circulation, and thus circulatory death, occurs within a short timeframe after withdrawal of cardio-respiratory supportive treatment (generally within 30 to 90 minutes), donated organs can be transplanted with successful outcomes.4 Best Practice Guideline for Donation after Circulatory Determination of Death (DCDD) in Australia Edition 1.0 October 2021, Australian Government Organ and Tissue Authority. ×
Situations where DCDD is considered include severe brain injury that has not and is not likely to progress to neurological death, end-stage cardio-respiratory or other organ failure, high spinal cord injury, and progressive neuro-muscular conditions.
Donation after Circulatory Death gives individuals and their families the opportunity to donate organs when neurological death hasn’t occurred, and provides additional organs for transplantation to the community. Currently, donors following a DCDD pathway comprise about 30% of organ donors in Australia and 16% of organ donors in New Zealand.6 There are, on average, fewer organs transplanted per donor via a DCDD versus a DNDD pathway, given the narrower organ suitability criteria that are applied in the situation of DCDD.6 ANZOD Registry. 2022 Annual Report, Section 1: Summary of Organ Donation and Transplant Activity. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2022. Available at: www.anzdata.org.au ×
Currently, approximately 30% of planned DCDD does not proceed to organ retrieval because death does not occur within the required time frames from withdrawal of cardio-respiratory support.7 Clinical practice improvements to refer all patients at end of life in the intensive care and emergency department settings has enhanced access to patients for potential donation via the DCDD pathway. This has continued to demonstrate an increased donation rate via this pathway.77 ANZOD Registry, 2022 Annual Report, Section 3: Deceased Oran Donor Pathway. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2021. Available at https://www.anzdata.org.au/anzod ×7 ANZOD Registry, 2022 Annual Report, Section 3: Deceased Oran Donor Pathway. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2021. Available at https://www.anzdata.org.au/anzod ×
2.1.3 Retrieval surgery
Each jurisdiction has processes in place to identify teams to undertake the surgical retrieval of abdominal or thoracic organs that have been assessed to be suitable for transplantation. Key team members from cardio- thoracic, liver or renal transplant units who will travel to the donor hospital may include surgeons, cardiac anaesthetists and perfusion technicians. Team members from the local hospital include theatre nursing staff, operating theatre technicians, anaesthetists and, sometimes, surgical assistants. The donation specialist coordinator also attends the retrieval surgery to coordinate the retrieval, assist with logistic arrangements, documentation of the process, support the theatre staff and care of the deceased post donation.
At surgical retrieval, organs are further assessed for suitability by retrieval surgeons in consultation with transplant surgeons and physicians. This may at times require adjunctive information such as the results of biopsies, which may not be available until after organ retrieval. Arrangements for the transportation of organs are made according to the organ type and whether organs are for local use or for transport interstate or between Australia and New Zealand.
There must be a reasonable prospect of at least one organ being transplantable before making the decision to proceed to retrieval surgery. The rate of non-utilisation of retrieved organs is expected to be small but greater than zero, since the final assessment of organ suitability can only be made at surgical retrieval. Information regarding organ quality and organ utilisation is collected and reviewed via the the ‘Organ Retrieval Report Form’ (ORRF).
2.2 Deceased donor and organ assessment
2.2.1 General evaluation of deceased organ donors
Organ suitability for transplantation is determined by the answers to two questions: (i) is the donor medically suitable to donate any organ, and (ii) is a particular organ suitable for transplantation.
Transplantation inevitably carries a small potential risk of transmission of infection or cancer from the donor to the recipient.8 That risk may vary depending on the organ and is assessed by considering donor risk factors and by testing the donor. Donor-derived disease transmission complicates less than 1% of all transplantation procedures (excluding Cytomegalovirus [CMV] and Epstein-Barr virus [EBV]) but can result in significant morbidity and mortality.9,10 While it is possible to quantify risks through screening and testing, the risks of transmission of infectious and other diseases cannot be completely eliminated.8 Kaul DR, Vece G, Blumberg E, La Hoz RM et al. Ten years of donor-derived disease: A report of the disease transmission advisory committee. Am J Transplant. 2021 Feb;21(2):689-702. ×9 Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant, 2011;11:1123–1130. 10 White SL, Rawlinson W, Boan P et al. Infectious disease transmission in solid organ transplantation: donor evaluation, recipient risk and outcomes of transmission. Transplantation Direct, 2018;4:e416. ×
The level of risk of disease transmission must be balanced against the risks to an individual patient of not proceeding with transplantation. The medical urgency of transplantation for some patients may mean that transplantation with an organ from a donor with increased risk of disease transmission is considered. Particularly where transplantation is life-saving, an increased risk of disease transmission may be regarded as acceptable to the recipient. Conversely, where transplantation is not immediately life-saving but instead aims to improve the quality of the recipient’s life, a greater margin of safety is appropriate. Nonetheless, transmission of infectious or other disease to recipients always remains a possibility, as there are limitations on diagnostic capabilities and limited time frames for donor assessment. It is important that the recipient has an informed view of accepting or rejecting an organ of lower quality and/or increased risk of disease transmission, with an understanding of the likely benefits from transplantation with the organ on offer (in terms of survival and/or quality of life), the likelihood of subsequent organ offers, and the risk of deterioration of their health status whilst waiting for an alternative offer. The conversation with the patient regarding consent to receive organs of lower quality or increased risk of disease transmission should occur early, ideally at the time of consent to waitlisting, and should be revisited periodically to take into account changes in patient priorities and health status.
Suitability of a particular organ for transplantation is influenced by a range of factors including donor age, size, medical history (including co-morbidities), lifestyle choices and specific organ size and pathology. The donation pathway will also influence organ suitability; that is, suitability will be affected by whether the donation was via a DCDD or DNDD pathway, the cold ischaemic time, the warm ischaemic time in case of DCDD, the surgical retrieval process, organ perfusion, organ storage and logistics.
It is increasingly possible to grade the quality of donated organs in order to provide a more accurate prediction of the medium and long-term functional outcomes of the organ post-transplantation. It is also possible to grade the risk of transmissible disease associated with a given donor and organ. This grading of organ quality and risk of disease transmission allows acceptance decisions to be tailored to individual recipients’ needs. That is, the potential benefit that is offered by a given organ may be insufficient for the needs of certain individuals (for example patients who are stable on medical therapy), however the same organ may increase the quality of life and survival prospects of other wait listed individuals (for example patients who are deteriorating on the waiting list or who are older).
2.2.2 Medical and social history
Obtaining a thorough medical, behavioural and travel history of the donor, performing a careful clinical examination and undertaking suitable investigations are critically important to the quality, safety and efficacy of organ donation. The accuracy of this information is critical to the assessment of the degree of risk to which the recipient of an organ from a given donor may be exposed. When interviewing next-of-kin and/or significant others regarding the history of a potential donor, it is important that this is done in a structured and standardised manner, utilising best practice tools such as the Australian Donor Risk Assessment Interview (AUS DRAI), to balance the rigorous requirements of screening with compassion, patience and empathy. In Australia, the donor’s medical history, examination and investigations are captured in an electronic donor record (EDR), which is completed for all donors, with the relevant information components provided to transplant units when organs are offered for transplantation. In New Zealand, the donor’s medical history, examination and investigations are captured in a Confidential Donor Referral (CDR), which is completed for all donors, with the relevant information components provided to transplant units when organs are offered for transplantation.
There are specific requirements for determining the suitability of each individual organ being considered for transplantation and these are identified in each organ-specific chapter. The general evaluation of donor suitability includes obtaining detailed information about the donor’s past medical and social history, paying particular attention to:
History of diseases and surgery, especially those that may affect organ function
History of diabetes, hypertension and other cardiovascular disease
Smoking, alcohol intake and non-medical drug use
History of tumours or cancer—including stage, pathology details, treatment and current status
Recent symptoms that may be indicative of undiagnosed infection, neurological disease or malignancy
Suggestion of underlying metabolic disorder
Risk factors for the transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), including non-medical injecting drug use, male to male sex, commercial sex work, time in prison, sex with a person at increased risk of these infections, or a young child of a mother at increased risk of these infections
Risk factors for the transmission of Transmissible Spongiform Encephalopathies (TSE), including family history of early dementia, use of pituitary hormone extract, notification of treatment with pituitary hormone extract
Place of birth and prior residence in countries outside of Australia and New Zealand
Travel history, especially recent travel (past six months)
History of animal contact
Information required regarding the donor’s current medical status and recent medical history includes:
Course of illness and cause of death
Vital signs and cardio-respiratory status, including mechanical and pharmacological supports
Function of potentially transplantable organs, including pathology, microbiological tests and imaging results
Surgery or other procedures
Medications
Administration of intravenous fluids and blood products (noting especially that haemodilution from large volume intravenous fluid may result in false negative serological test results).
There are very few absolute exclusion criteria to organ donation, with the exception of disseminated metastatic cancer and donors with known specified factors for TSE (see Section 2.3.5.1). All other risk factors should be interpreted in the context of all other donor characteristics and recipient factors.
2.2.3 Physical examination
Physical examination provides information relevant to suitability, allocation, and possible disease transmission risks. This should include:
Height and weight
General assessment with respect to body habitus and state of health, major abnormalities related to past or present disease (e.g. obvious limb ischaemia, chest or spinal deformities, traumatic injuries)
Inspection of skin, including the skin of the back and careful examination in skin folds and around the genital and anal areas, looking for surgical scars, skin lesions indicating possible cancers or infections, injection sites/needle track marks suggesting intravenous drug use (IVDU), or lumps, sores, tattoos, rashes or mole irregularity
Look for obvious abnormalities, lumps or masses (e.g. neck, groin, axillae, breasts, abdomen).
An additional physical examination by an experienced surgeon(s) at the time of retrieval is also important, as this may reveal unexpected clinically occult lesions such as bowel cancers or renal or liver tumours.
2.2.4 Laboratory investigations
Blood group for ABO and Rhesus are mandatory investigations for all donors. For women of child-bearing potential dying from unexplained intracerebral haemorrhage, testing for beta human chronic gonadotrophin hormone is recommended to detect metastatic choriocarcinoma. Whilst routine post-mortem examination has become an uncommon procedure in clinical medicine, if an autopsy is performed then the results should be followed-up by the donation service and communicated back to the relevant transplanting units as the autopsy may detect potentially transmissible disease.
The list of possible pathogens for which potential donors might be screened is very long. Screening of these pathogens depends on whether:
The pathogen is sufficiently prevalent in the population that screening would be useful
There is evidence that the pathogen in question can be transmitted by organ transplantation
Transmission of the pathogen could result in significant morbidity or mortality
A sufficiently accurate, rapid and affordable screening test exists.
The rapid turn-around times necessary in the context of donor screening, the associated logistical and technical limitations, and the need to balance the risk of transmission of infection against the risks to the recipient of dying while awaiting transplantation, make the goals of screening potential organ donors different to screening blood or tissue donors. It is the goal of organ donation and transplantation programs to minimise unexpected infectious disease transmission events while simultaneously maximising opportunities for transplantation. All infectious disease screening recommendations, therefore, carefully consider turn-around times, test performance (i.e. the potential for false positive or false negative results), and other logistical issues that may pose a risk to the donation process and lead to the loss of transplantable organs. These considerations must be weighed against the benefits of screening to patient safety.
The following laboratory investigations to detect infections that may be transmitted by solid organ transplantation are recommended for all donors:
HIV antigen/HIV-1/2 antibody combination assay (HIV Ag/Ab) • Hepatitis B surface antibody (anti-HBs or HBsAb)
Hepatitis B surface antigen (HBsAg)
Hepatitis B core antibody (HBcAb)
Hepatitis C antibody (anti-HCV or HCV Ab)
Nucleic acid testing (NAT) for HBV, HCV and HIV, most commonly using polymerase chain reaction (PCR) assays
Cytomegalovirus (CMV) immunoglobulin (IgG) antibody
Epstein-Barr virus (EBV) capsid IgG antibody
Syphilis serology (specific treponemal antibody test)
Toxoplasmosis serology (IgG)
Human T-cell-lymphotrophic virus (HTLV) 1/2 antibody
Strongyloides stercoralis serology (IgG).
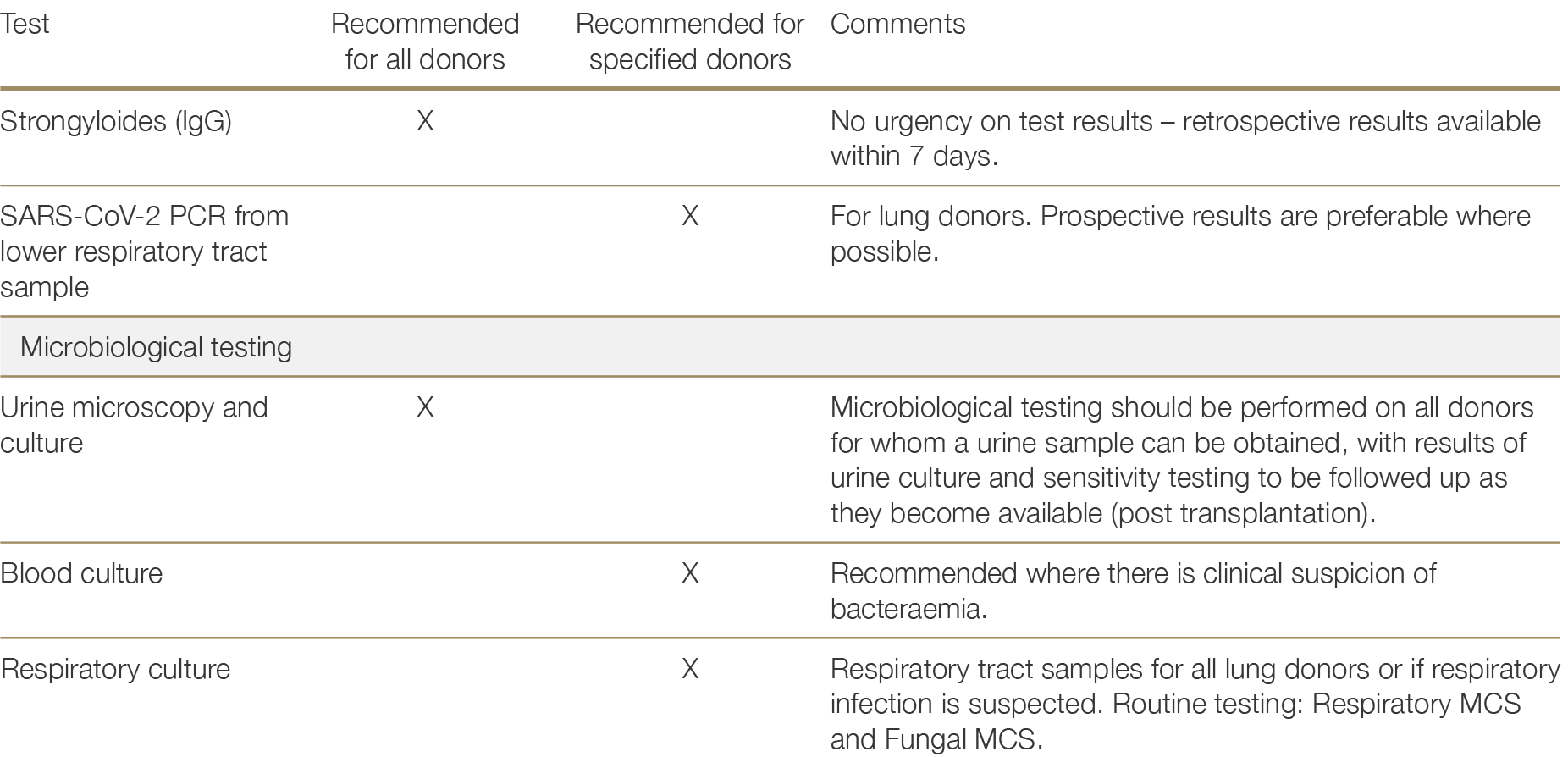
Urine microscopy and culture is recommended for all donors from whom a urine sample can be obtained, with the results of cultures and sensitivity testing to be followed up as soon as they become available (which may not be until after transplantation has occurred). Blood cultures are recommended only if there is clinical suspicion of bacteraemia. A respiratory tract sample (i.e; endotracheal aspirate, sputum or bronchoscopic sample), bacterial culture, fungal culture and SARS-CoV-2 PCR is recommended for all lung donors.
Diagnostic testing for tuberculosis is only recommended where there is suspicion of tuberculosis infection that is supported by epidemiological and clinical factors (see Section 2.3.3.5).
Table 2.1 provides details of which donors should receive the tests specified above and whether results are recommended prospectively. “Prospective results” in the context of organ donation refers to results that are made available prior to the transplantation of organs (as opposed to prior to organ retrieval). Test results that are not recommended to be made available prospectively should be obtained as early as possible, but transplantation may proceed prior to results being available.
Table 2.1: Recommended laboratory investigations for the detection of potentially transmissible infectious diseases in solid organ donors.
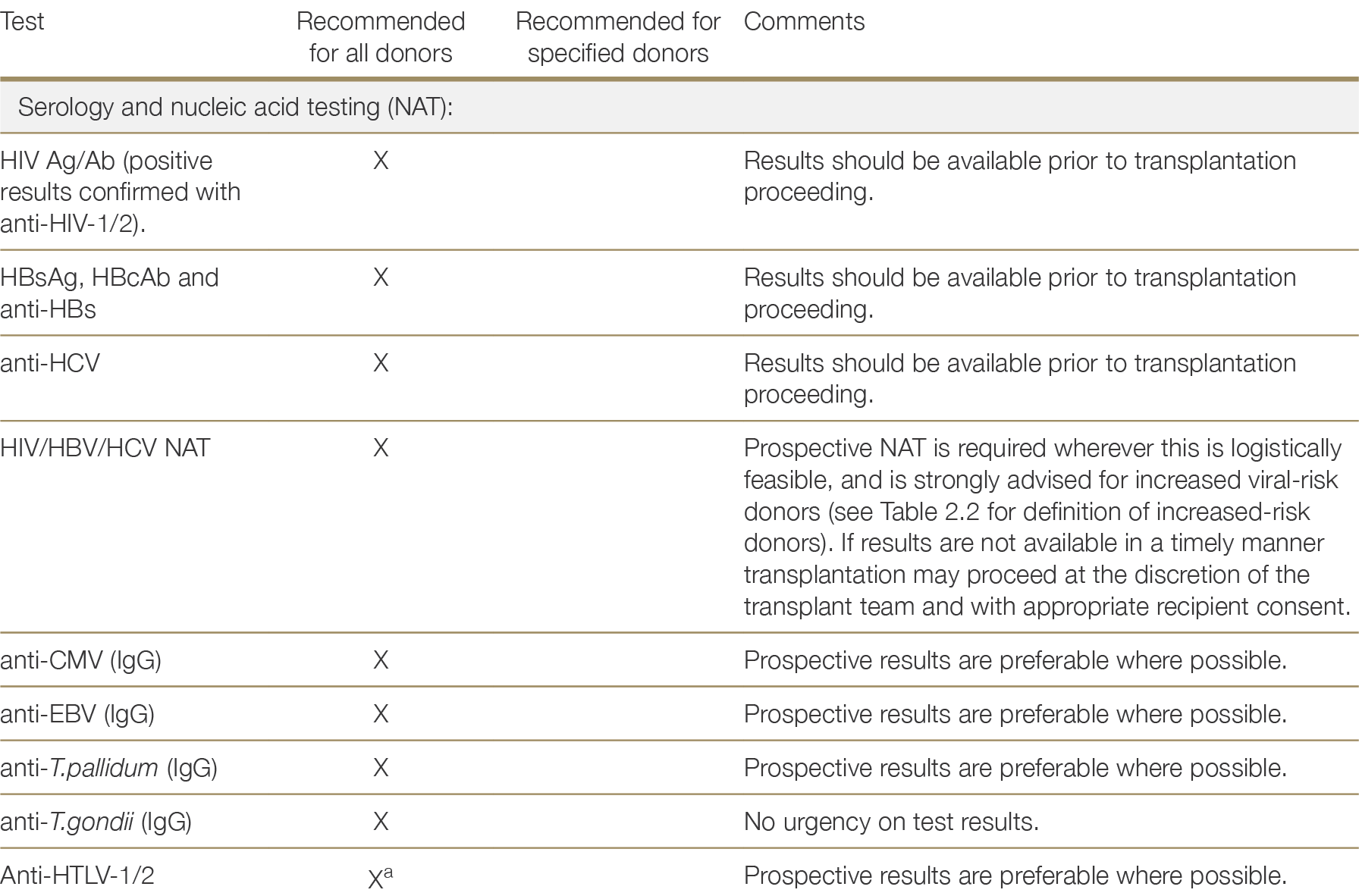
a While HTLV-1/2 screening is recommended for all donors, donors at high risk of HTLV-1 include Aboriginal people from Central Australia and persons born in southwestern Japan, sub-Saharan Africa, the Middle East, the Caribbean, and parts of South America (French Guyana, Peru). Screening is recommended for all donors since information in the donor record might not identify all persons at high risk and outcomes in the rare event of transmission can be extremely severe or fatal. See Section 2.3.2.9.a No reference text available.×
2.2.5 Haemodilution assessment
Where the donor receives multiple blood transfusions or significant infusions of intravenous fluids prior to donation, haemodilution may occur such that circulating antigens, antibodies and targets for NAT are at a low concentration that is difficult to detect, introducing the potential for false negative results. False positive results may also occur due to interactions between serological tests and molecules present as a result of infused products. The degree to which a potential donor’s plasma has been diluted is a product of blood loss as well as fluids infused.
Serological tests and NAT have not been validated for use on all haemodiluted samples, and therefore serological screening and NAT should ideally be performed on non-diluted blood samples. For all donors, blood products and colloids given in the 48 hours prior to the date and time the sample was drawn are entered into the EDR (Australia) or CDR (New Zealand). This information is used to autocalculate whether the sample is haemodiluted. If either plasma dilution or blood dilution exceed defined thresholds, a pre-transfusion/ infusion sample should be used for donor screening. If a suitable sample is not available, the risk of false negative results from testing a haemodiluted sample should be communicated to the transplanting teams.
2.2.6 Special donor groups
Donors under 18 months or breastfed children
Microbiological screening for neonatal and infant donors (of less than 18 months old, or up to 6 months beyond breast feeding) should be performed as for other donors, including HIV/HBV/HCV NAT, taking into account that positive antibody results may reflect passive transfer of antibodies from the mother. The potential for eclipse/ window period infections should also be considered, and prospective NAT is recommended in this context.
Given the limited volume of blood that can be taken from a neonate or infant for the purposes of screening and the likelihood of haemodilution, complementary testing of the mother is required in these cases. If the mother is not at increased-risk of infectious diseases (see Table 2.2) and is sero-negative for markers of infection, the successful screening of the neonate/infant is less critical. For mothers who are deemed an increased viral risk, discussion with an infectious diseases physician or microbiologist is strongly advised.
2.3 Risk of donor transmitted infectious disease
Acute or latent infections may be transmitted by the transplanted organ to the recipient. The intentional use of donors with certain infections may be considered where there is an acceptable risk of morbidity to the recipient, mitigated by serostatus matching, antimicrobial treatment or prophylaxis and/or monitoring. The unexpected transmission of an infectious disease from an organ donor to recipient(s) is a rare event; however, when it does occur, it is usually associated with significant morbidity and mortality.99 Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant, 2011;11:1123–1130. ×
Donor history, examination and testing both reduce the risk of unexpected infection transmission and also inform risk-stratification where donors carry an increased risk of disease transmission. For instance, close attention must be paid to travel history: potential donors with recent travel to or previous residence in areas where they may have been exposed to endemic pathogens warrant additional screening.10,1110 White SL, Rawlinson W, Boan P et al. Infectious disease transmission in solid organ transplantation: donor evaluation, recipient risk and outcomes of transmission. Transplantation Direct, 2018;4:e416. 11 Ison, M.G., P. Grossi, and A.S.T Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant, 2013. 13 Suppl 4: p. 22-30. ×
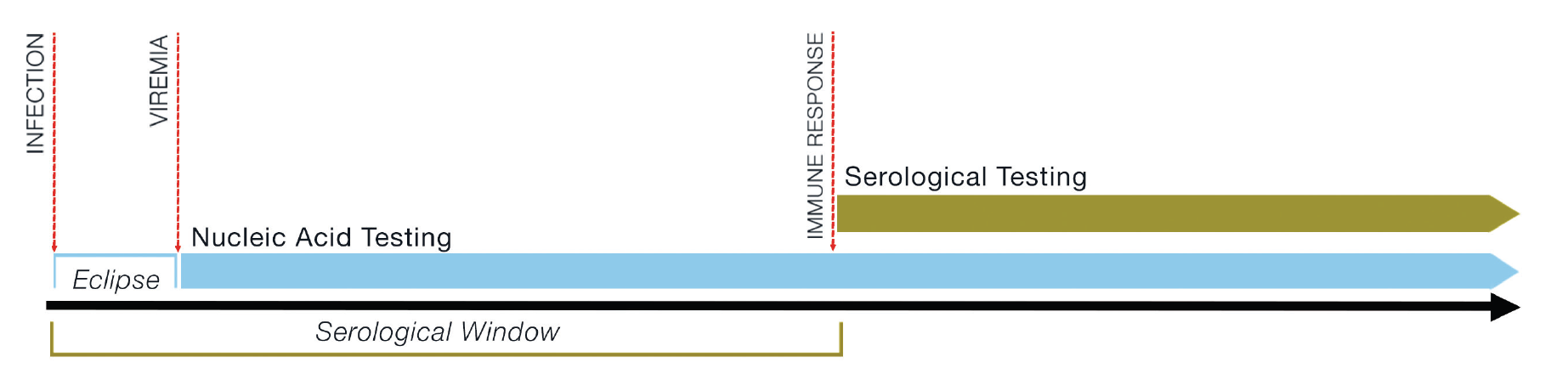
In addition, the concept of the “eclipse” or “window” period of infection is critical to understanding donor infectious disease risk mitigation. Following infection by a microbiological agent, there is a period of time during which no microbe can be readily detected in the host; this is called the “window period” for serological testing, or the “eclipse period” for NAT (see Figure 2.1). Unexpected transmissions are most likely to occur if the donor has recently acquired the infection and is still in the eclipse/window period before detection is possible. As such, test results must be interpreted in the context of the patient’s full history, and the probability of false negative results needs to be considered against the donor’s background of any reported risk factors such as IVDU or high-risk sexual contact.
Figure 2.1: Generalised diagram of eclipse and window periods.
2.3.1 Donors at increased risk of HIV, HBV and HCV
The unexpected transmission of HIV, HCV or HBV through transplantation is rare, particularly in the setting of thorough patient history, examination and laboratory testing (serology and NAT). While it is important to stratify donor risk of blood-borne virus acquisition, increasingly organs from otherwise suitable donors with increased risk for HIV, HCV or HBV infection are being used to expand the donor pool both in Australia and overseas. Recipients of increased risk donor organs have similar overall and graft survival compared to recipients from other donors.12,13,14 Organ recipients in the United States who declined donors with increased risk had worse long-term survival than recipients who accepted increased risk donor, probably due to prolonged waiting times.15,16,1712 Kizilbash SJ, Rheault MN, Wang Q et al. Kidney transplant outcomes associated with the use of increased risk donors in children. Am J Transplant. 2019 Jun;19(6):1684-1692. 13 Xie MW, Kennan SP, Slaunwhite A, Rose C. Observational Study Examining Kidney Transplantation Outcomes Following Donation From Individuals That Died of Drug Toxicity in British Columbia, Canada. Can J Kidney Health Dis. 2023 Apr 4;10:20543581231156853. 14 Okoh AK, Chan O, Schultheis M et al. Association between increased-risk donor social behaviors and recipient outcomes after heart transplantation. Clin Transplant. 2020 Mar;34(3):e13787 ×15 Bowring MG, Holscher CM, Zhou S et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant. 2018 Mar;18(3):617-624. 16 Cox ML, Mulvihill MS, Choi AY et al. Implications of declining donor offers with increased risk of disease transmission on waiting list survival in lung transplantation. J Heart Lung Transplant. 2019 Mar;38(3):295-305. 17 Mulvihill MS, Cox ML, Bishawi M et al. Decline of Increased Risk Donor Offers on Waitlist Survival in Heart Transplantation. J Am Coll Cardiol. 2018 Nov 6;72(19):2408-2409. ×
Donors are classified as “increased risk” based on the presence of any of the risk factors listed in Table 2.2. In reality, the risk of unexpected donor-derived transmission of HIV, HBV and/or HCV exists on a spectrum, and varies by the type of risk behaviour, recency of behaviour and any risk-mitigation employed. It should be noted that information about behavioural risk factors obtained from the next of kin may be limited or inaccurate. The donor assessment interview must be supplemented with evidence from physical examination of the donor and/ or their medical record. Donors whose social and medical history cannot be obtained should also be treated as increased risk.
The presence of HIV, HBV or HCV in the donor is not a contraindication to donation. Decisions about whether to proceed with donation and transplantation will depend on recipient informed consent, the nature of the infection, other recipient clinical factors and the availability of effective treatment. The presence of HIV usually rules out donation, with exceptions including for recipients living with HIV., similarly, recipients who are adequately immunised against or given prophylactic treatment for HBV may be transplanted with organs from donors with the potential to transmit HBV. The presence of HCV in a donor is no longer a barrier to transplantation given the availability of curative treatment. See Sections 2.3.2.4 and 2.3.2.5 for more detail.
Donor testing for HIV, HBV and HCV using serology and NAT should be undertaken using blood samples obtained from the donor prior to significant haemodilution. Such testing should be undertaken by laboratories with the appropriate accreditation (National Association of Testing Authorities [NATA] and Royal College of Pathologists of Australia [RCPA] or Therapeutic Goods Administration [TGA, licensed]). Serological testing for HIV, HBV and HCV is performed as part of the evaluation of all donors, with results obtained prior to proceeding with organ transplantation. NAT testing for HIV, HBV and HCV is also recommended for all donors, with results required prospectively wherever logistically feasible.
Table 2.2: Criteria for identifying organ donors at increased risk for HIV, HBV, and HCV infection (MSM= men who have sex with men; derived from Jones18)18 Jones JM, Kracalik I, Levi ME et al. Assessing Solid Organ Donors and Monitoring Transplant Recipients for Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Infection - U.S. Public Health Service Guideline, 2020. MMWR Recomm Rep. 2020 Jun 26;69(4):1-16. ×

* 30 days represents the maximum eclipse period for detection of HIV, HBV, and HCV via NAT.
If a donor has recently been infected with HIV, HBV or HCV, it is possible that the donor may still be in the eclipse or window period of infection (see Table 2.3) and transmission may still occur despite negative results on serology and NAT. The degree of residual infection risk associated with a specific donor is influenced by the nature of the donor’s risk behaviour(s) and how recently the risk behaviour(s) occurred in relation to the time of donor testing.19 Higher underlying incidence in an at-risk group or longer eclipse/window periods correspond with a higher residual risk of an undetected infection.19 Ison MG. Nucleic Acid Testing of Organ Donors: Is the Glass Half Empty or Half Full? Am J Transplant 2015;15:1743–174. ×
Table 2.3: Window and eclipse periods* for pathogen testing. Modified from Humar.2020 Humar A, Morris M, Blumberg E, et al. Nucleic acid testing (NAT) of organ donors: is the “best” test the right test? A consensus conference report. Am J Transplant 2010;10:889-99. ×

* Window period = the interval from infection to ability to detect that infection by serological testing; eclipse period = the interval after infection for which infection cannot be detected by either NAT or serological testing
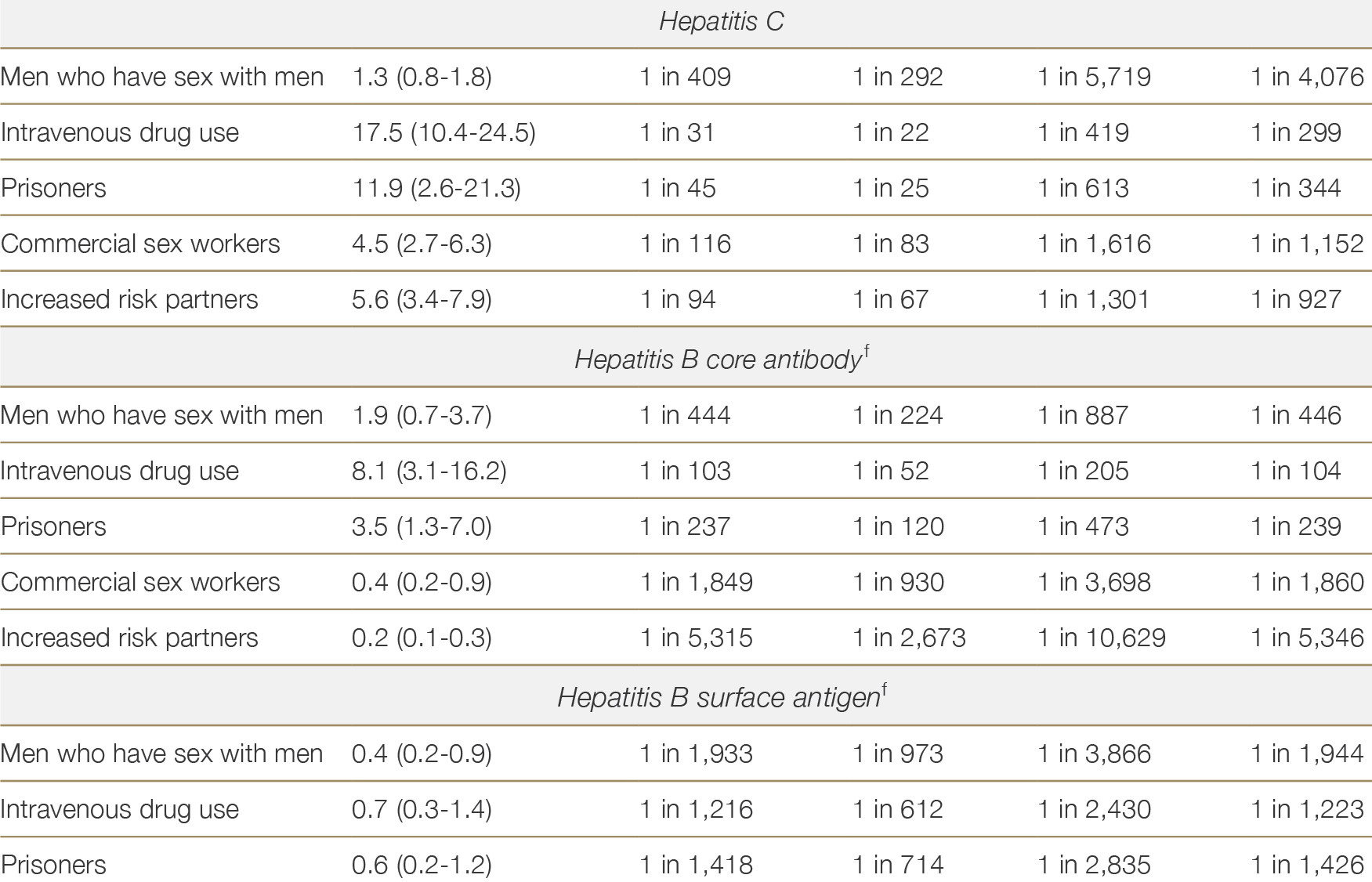
Table 2.4 lists the estimated risks of undetected HIV, HBV or HCV infection in Australian donors by risk behaviour type, based on Australian epidemiological data.21 These estimates of residual risk are based upon the best available local evidence, but are limited where the underlying data were sparse – notably in the case of commercial sex workers and high-risk partners. Data on the incidence of HBV in Australia is limited, therefore residual risk estimates were derived from estimates of the prevalence of hepatitis B core antibody (HBcAb) and hepatitis B surface antigen (HBsAg) in each risk group. It is also important to note that these residual risk estimates are based on historical data. Since 2019 when the residual risks in Table 2.4 were calculated, widespread uptake of HIV pre-exposure prophylaxis among MSM, and direct acting antiviral therapy for HCV, have led to reductions in the prevalence and incidence of these infections among their highest risk groups, and the stated estimates are likely to be over-estimates.22,23,24,2521 Waller K, de la Mata N, Wyburn K et al. Residual risk of blood borne virus infection when Australian organ donor referrals test negative: a systematic review and meta-analysis. Med J Aust 2019, 211 (9): 414-420. ×22 Callander D, McManus H, Gray RT et al. HIV treatment-as-prevention and its effect on incidence of HIV among cisgender gay, bisexual, and other men who have sex with men in Australia: a 10-year longitudinal cohort study. Lancet HIV. 2023 Jun;10(6):e385-e393. 23 Iversen J, Wand H, McManus H et al. Incidence of primary hepatitis C virus infection among people who inject drugs in Australia pre- and post-unrestricted availability of direct acting antiviral therapies. Addiction. 2023; 118: 901–11. 24 Dutch MJ, Seed CR, Cheng A et al. Recently Acquired Blood-borne Virus Infections in Australian Deceased Organ Donors: Estimation of the Residual Risk of Unexpected Transmission. Transplant Direct. 2023 Feb 17;9(3):e1447. 25 Dutch MJ, Patrick CJ, Boan PA et al. Prevalence of Blood-Borne Viruses and Predictors of Risk in Potential Organ Donors in Australia. Transpl Int. 2022 May 3;35:10395. ×
As predicted by residual risk estimates, unexpected blood- borne virus (BBV) transmission is uncommon. The US CDC 2014-2017 investigated instances where recipient HBV or HCV NAT was positive within 18 months of transplantation, where donors were serology and NAT negative. There were 7 HBV NAT positive recipients, 5 were liver transplant recipients and 6 survived with functioning grafts. There were 20 HCV NAT positive recipients, 19 survived, 18 with functioning grafts. HBV was diagnosed 120-450 days and HCV at 20-190 days post-transplant. All donors were classified as increased risk, 70% had used intravenous drugs.26 There are only a few occurrences of unintentional HIV donor derived infection in the literature since donors have been routinely tested with NAT and serology, mostly due to miscommunication of donor results.2726 Bixler D, Annambhotla P, Montgomery MP et al. Unexpected Hepatitis B Virus Infection After Liver Transplantation - United States, 2014-2019. MMWR Morb Mortal Wkly Rep. 2021 Jul 9;70(27):961-966. ×27 Pereira MR, Dube GK, Tatem L, Burack D, Crew RJ, Cohen DJ, Ratner LE. HIV transmission through living donor kidney transplant: An 11-year follow-up on the recipient and donor. Transpl Infect Dis. 2021 Aug;23(4):e13691. ×
The risk of an undetected HIV infection is low. Donors with the highest residual risk, men who have recently had sex with men, have an estimated 1 in 1621 residual risk of undiagnosed HIV based on a negative enzyme immunoassay (EIA) result alone, and a 1 in 5092 residual risk based on a negative EIA + NAT. For recent intravenous drug users, prisoners, commercial sex workers and increased risk partners, the risk of undiagnosed HIV is less than 1 in 10,000.
It should be noted that the underlying risk behaviours within each risk factor category are not homogenous. The residual risks reported in Table 2.4 represent conservative estimates of the infectious risks associated with donors in each risk category; however, the actual risk of undetected infection in a given test-negative donor may be significantly lower depending on their history. For example, residual risks of HCV among IVDU may be lower for IVDU participating in needle exchange programs and receiving opioid substitution, compared to IVDU not participating in these programs.28 For all donors, test results should be interpreted in the context of the donor’s personal history and the residual risk estimates given in Table 2.4 should be used as a guide but not as definitive numbers.28 Dutch MJ, Armstrong EJ, Malcher KJ and Allan WB. Risk of hepatitis C transmission from elevated risk organ donors in Australia is low: implications for routine referral of potential donors. Presentation to the Australian and New Zealand Intensive Care Society Annual Scientific Meeting, Adelaide, 2018. ×
Table 2.4: Residual riska of undiagnosed HIV, HBV or HCV infection for Australian donors at increased risk, by risk factor and testing strategy. Adapted from Waller et al.21a No reference text available.×21 Waller K, de la Mata N, Wyburn K et al. Residual risk of blood borne virus infection when Australian organ donor referrals test negative: a systematic review and meta-analysis. Med J Aust 2019, 211 (9): 414-420. ×
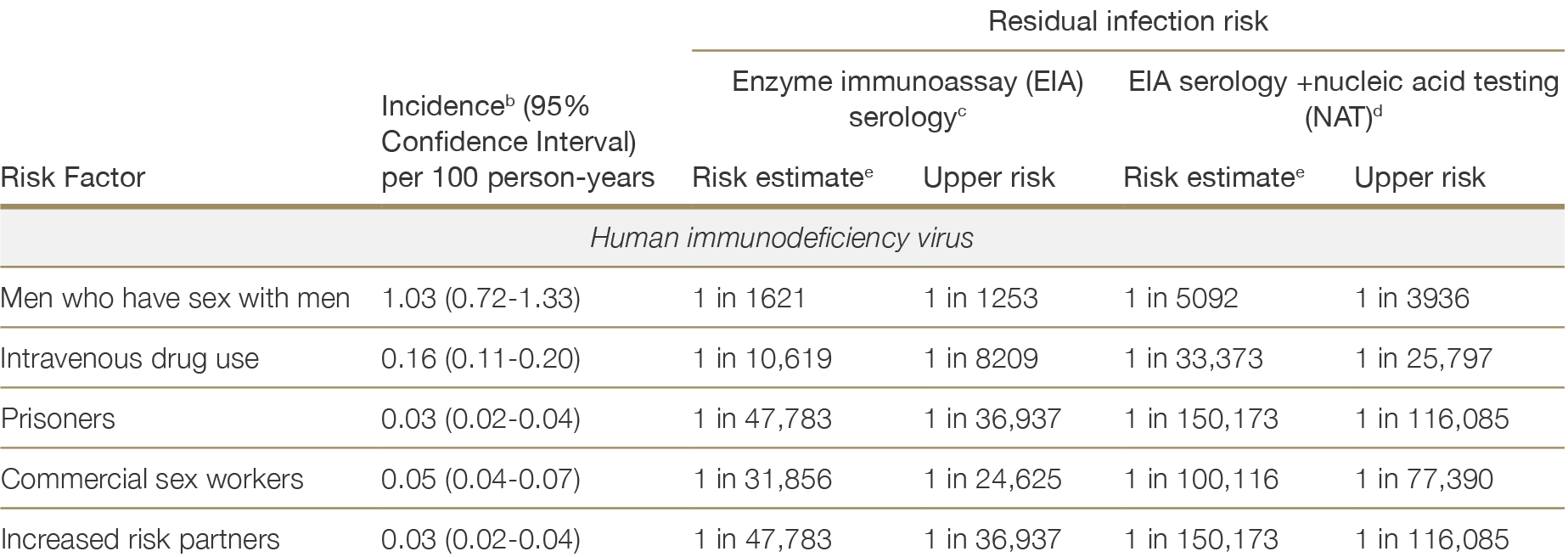
a Residual infection risk is the predicted rate of undetected infection in donors who test negative for HIV, HCV or HBV, depending on risk factor and testing strategy, calculated as RR = 1 – e(-incidence)*(eclipse period or serological window)a No reference text available.×
b Incidence estimates are based on a systematic review and meta-analysis of studies from 2000-2017 reporting original estimates of Australian HIV, HCV or HBV prevalence or incidence. Incidence rates and confidence intervals were estimated using random effects.b No reference text available.×
c Serological window period assumed in the calculation of residual risk estimates based on serological screening (EIA) alone: HIV=22 days, HCV=70 days, HBV=44 days20c No reference text available.×20 Humar A, Morris M, Blumberg E, et al. Nucleic acid testing (NAT) of organ donors: is the “best” test the right test? A consensus conference report. Am J Transplant 2010;10:889-99. ×
d Eclipse period for NAT testing assumed in the calculation of residual risk estimates based on EIA + NAT: HIV=7 days, HCV=5 days, HBV=22 days.20d No reference text available.×20 Humar A, Morris M, Blumberg E, et al. Nucleic acid testing (NAT) of organ donors: is the “best” test the right test? A consensus conference report. Am J Transplant 2010;10:889-99. ×
e Upper risk estimate is derived from the upper 95% confidence limit of the risk estimate.e No reference text available.×
f Data on the incidence of HBV in the Australian population are not available. It was therefore necessary to estimate the residual risk of undetected HBcAb and HBsAg separately. These estimates should be interpreted as the risk that, despite a negative test result, the donor is positive for either HBcAb (past, persistent or acute-phase infection) or HBsAg (active infection) respectively.f No reference text available.×
General considerations when transplanting organs from increased risk donors or donors with BBV
From an increased viral risk donor, the transmission risk is highest for HCV, which is now highly treatable after transplant, leading many units globally to routinely transplant organs from HCV NAT positive donor to HCV NAT negative recipients. The risk of HBV transmission is low, appears to occur predominately through liver transplantation, and is treatable. The risk of HIV transmission is very low. For these reasons routine use of organs from increased viral risk donors can occur, with informed consent and ensuring prospective donor NAT testing and routine testing of recipient post-transplant.
Follow-up of recipients of organs from increased viral risk donors
For all recipients of organs from donors identified by transplant clinicians as being at increased risk of infection with HIV, HBV or HCV, post-transplant surveillance for the appearance of infection should occur. NAT testing is required for HCV and preferred for HBV and HIV where possible; alternatives for the latter viruses are HBsAg and HIV antigen/antibody serological testing. Recommendations are for:
one-time testing at 4-6 weeks post-transplantation
HBV and HCV testing in the investigation of liver injury
HBV testing at 1 year for liver recipients
Immediate verbal communication with the relevant donation agency needs to occur and subsequent documentation if testing indicates de novo infection with HIV, HBV or HCV in the follow-up period post transplantation.
2.3.2 Viral Infections
2.3.2.1 Coronavirus (SARS-CoV-2) causing COVID-19
Transmission of SARS-CoV-2 has not been documented via transplantation of organs from SARS-CoV-2 PCR positive donors, other than lung. Non-lung allograft outcomes appear equivalent for recipients of organs from SARS-CoV-2 PCR positive and negative donors alike.29,30 Data on outcomes of recipients receiving lung transplantation from a donor testing positive by PCR for SARS-CoV-2 are limited to small cohort studies, with limited granular data regarding details of donor infection and/or recipient management.31,32,33 SARS-CoV-2 PCR positive lung donation has occurred safely under specific circumstances and outcomes are expected to be better where there is no evidence of significant pneumonitis, where SARS-CoV-2 infection is in later stages (e.g. “weak” PCR results, higher CT value), where the recipient is immune from vaccination/prior infection and otherwise has a good, predicted outcome from lung transplantation.29 Schold JD, Koval CE, Wee A et al. Utilization and outcomes of deceased donor SARS-CoV-2-positive organs for solid organ transplantation in the United States. Am J Transplant. 2022 Sep;22(9):2217-2227. 30 Wolfe SB, Singh R, Paneitz DC et al. One Year Outcomes Following Transplantation with COVID-19-Positive Donor Hearts: A National Database Cohort Study. Journal of Cardiovascular Development and Disease. 2024; 11(2):46. ×31 Asija R, Singh R, Paneitz DC et al. Is Transplantation with Coronavirus Disease 2019-Positive Donor Lungs Safe? A US Nationwide Analysis. Ann Thorac Surg. 2023 Nov;116(5):1046-1054. 32 Hwang J, Yuen A, Rhoades J et al. Real-time transcription polymerase chain reaction cycle threshold values as criteria for utilization of incidental COVID 19 positive lung donors. J Heart Lung Transplant. 2023 Mar;42(3):301-304. 33 Schroder J, Bryner BS, Spencer PJ et al. Transplanting thoracic COVID-19 positive donors: An institutional protocol and report of the first 14 cases. J Heart Lung Transplant. 2022 Oct;41(10):1376-1381. ×
Recommendations
Donation can proceed from non-lung donors with positive SARS-CoV-2 PCR provided the transplanting organ has not been damaged by the infection. Lung transplantation from SARS-CoV-2 PCR positive donor can be considered on a case-by-case basis.
2.3.2.2 Cytomegalovirus
Over 50% of the Australian adult population is latently infected with cytomegalovirus (CMV), based on rates of seropositivity in population studies.34 No contraindications exist to organ donation in the case of latent CMV. However, organs from seropositive donors may transmit infection, potentially causing severe disease in the seronegative recipient.3534 Seale H, MacIntyre CR, Gidding HF, et al. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol, 2006; 13(11):1181. ×35 Kotton, Camille N, Kumar, Deepali, Caliendo, Angela M et al. The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 102(6):p 900-931, June 2018 ×
De novo CMV infection in the recipient can be largely managed by routine prophylaxis and post-transplant virological monitoring. Selecting CMV seronegative donors for CMV negative recipients avoids de novo CMV infection, however in practice there are often competing interests to seromatching.
Recommendation
Organs can be accepted irrespective of the CMV serostatus of the donor. If the donor or recipient is seropositive, suitable prophylaxis should be given and post-transplant virological monitoring is required.
2.3.2.3 Epstein-Barr virus
Over 90% of Australian adults are latently infected with Epstein-Barr virus (EBV).36 Epstein Barr virus causes lifelong infection, and organs from seropositive donors may transmit infection to a seronegative recipient, increasing the risk of post-transplant lymphoproliferative disease (PTLD). The risk of PTLD is approximately six-times higher in cases of donor-derived primary EBV infection versus cases of EBV reactivation in seropositive recipients.3736 Lai PK, Mackay-Scollay EM and Alpers MP. Epidemiological studies of Epstein-Barr herpesvirus infection in Western Australia. J Hyg, 1975; 74(3):329-37. ×37 Sampaio MS, Cho YW, Shah T, et al. Impact of Epstein-Barr virus donor and recipient serostatus on the incidence of post- transplant lymphoproliferative disorder in kidney transplant recipients. Nephrol Dial Transplant, 2012; 27(7): 2971-9. ×
Antiviral prophylaxis has not been shown to reduce the incidence of PTLD, therefore monitoring for the appearance of EBV deoxyribonucleic acid (DNA) and early treatment should be considered for all donor-positive/ recipient-negative (D+/R-) transplants. In cases of suspected acute mononucleosis in the donor, diagnosis should be made on the basis of investigation of EBV-DNA in peripheral blood and EBV nuclear antigen.
Recommendation
Organs can be accepted irrespective of the EBV serostatus of the donor. If the donor is seropositive and the recipient seronegative, post-transplant virological monitoring is suggested.
2.3.2.4 Hepatitis B virus
Screening for hepatitis B virus (HBV) in potential organ donors uses hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBcAb) and hepatitis B surface antibody (HBsAb) to distinguish between current infection, prior cleared infection, vaccination, or no exposure.38 HBV nucleic acid testing (NAT) is also recommended for all donors as occult HBV infection may occur. This is generally available prior to transplantation, but if NAT results are not yet available, the pathway for a NAT +ve donor should be followed (see Figure 2.2). Recipient immunity to HBV is usually defined by HBsAb titre >10IU/L, although weak evidence suggests possible additional benefits of titres >100IU/L where an increased risk of HBV transmission exists in the post-transplant setting.39,40 Table 2.5 below summarises the interpretation of donor HBV screening and recommendations for utilisation. Figure 2.2 provides a further decision flow framework for the interpretation of HBV test results and utilisation of organs from HBV infected donors.38 Natov, S.N. and B.J. Pereira. Transmission of viral hepatitis by kidney transplantation: donor evaluation and transplant policies (Part 1: hepatitis B virus). Transpl Infect Dis, 2002. 4(3): p. 117-23. ×39 Chancharoenthana W, Leelahavanichkul A, Udomkarnjananun S, et al. Durability of Antibody Response Against the Hepatitis B Virus in Kidney Transplant Recipients: A Proposed Immunization Guideline From a 3-Year Follow-up Clinical Study. Open Forum Infect Dis. Jan 2019;6(1):ofy342. doi:10.1093/ofid/ofy342 40 Celebi ZK, Sengul S, Soypacaci Z, et al. Kidney transplantation from hepatitis B (HB)-positive donors to HB negative recipients: anti-HB Core immunoglobulin G became positive in all recipients after the transplantation. Transplant Proc. Apr 2013;45(3):923-5. doi:10.1016/j.transproceed.2013.02.058 ×
HBsAg-positive and/or HBV NAT-positive donors
HBsAg-positive and/or HBV NAT-positive donors are considered to have active HBV infection and pose a transmission risk.41,42 Donors who are HBsAg-negative and HBV NAT-positive likely have occult HBV infection, typically with low HBV DNA viral load, but still pose a transmission risk. The risk of HBV transmission is lower where donor HBV DNA is undetectable, among non-liver organ recipients, recipients with a protective HBsAb titre, and with use of antiviral prophylaxis.41,42,4341 Jiang H, Wu J, Zhang X, et al. Kidney Transplantation from Hepatitis B Surface Antigen Positive Donors into Hepatitis B Surface Antibody Positive Recipients: A Prospective Nonrandomized Controlled Study from a Single Center. Am J Transplant, 2009;9(8):1853-1858. 42 Wei HK, Loong CC, King KL, et al. HBsAg(+) donor as a kidney transplantation deceased donor. Transplant Proc, 2008;40(7):2097-9. ×41 Jiang H, Wu J, Zhang X, et al. Kidney Transplantation from Hepatitis B Surface Antigen Positive Donors into Hepatitis B Surface Antibody Positive Recipients: A Prospective Nonrandomized Controlled Study from a Single Center. Am J Transplant, 2009;9(8):1853-1858. 42 Wei HK, Loong CC, King KL, et al. HBsAg(+) donor as a kidney transplantation deceased donor. Transplant Proc, 2008;40(7):2097-9. 43 Chung RT, Feng S, and Delmonico FL. Approach to the Management of Allograft Recipients Following the Detection of Hepatitis B Virus in the Prospective Organ Donor. Am J Transplant, 2001;1(2):185-191. ×
HBsAg-positive and/or NAT-positive donors can be used for HBsAg-positive recipients (D+/R+), among whom lifelong antiviral therapy is routinely recommended regardless of donor HBV status.4444 Pilmore HL and Gane EJ, Hepatitis B-positive donors in renal transplantation: increasing the deceased donor pool. Transplantation, 2012;94(3):205-10. ×
Additionally, the hepatitis D virus (HDV) status of the donor should be determined, including HDV ribonucleic acid (RNA) and HDV antibody assays. The results of these assays will often not be available until after transplantation. Where there is a suspected or known risk of HDV transmission, transplantation should be discussed with an infectious diseases physician or hepatologist prior to proceeding
Liver recipients
While HBsAg D+/R- liver transplants have been performed after exclusion of donor liver fibrosis45,46 and can be considered in specific circumstances, there are increased risks in this setting, including the unproven but potential increased risk of hepatocellular carcinoma. Nonetheless, these transplants may be appropriate for some recipients. Lifelong antiviral therapy is recommended.45 Xu G, Jiang C-H, Xiao Y, et al. Utilization of hepatitis B virus-positive allografts in liver transplantation: a new arrow to the bowstring for expanding the donor pool? Hepatobiliary Surgery and Nutrition. 2022;11(2):283-287. 46 Delman AM, Turner KM, Safdar K, et al. Expanding the Donor Pool: First Use of Hepatitis B Virus Nat Positive Solid Organ Allografts Into Seronegative Recipients. Ann Surg. Oct 1 2021;274(4):556-564. doi:10.1097/sla.0000000000005071 ×
Non-liver recipients
Non-liver organs from HBsAg-positive and/or NAT-positive donors may also be used for HBsAg-negative recipients (D+/R-), with appropriate precautions and consent. Non-liver organs from donors who are HBsAg-positive/HBV NAT-positive have safely been used for HBsAg-negative recipients (D+/R-) in a number of cohorts,47,48 including from deceased donors in non-endemic countries analogous to Australia.46,49 Hepatitis B immunoglobulin can be used at the time of transplant for non-immune recipients. Entecavir or tenofovir are the optimal antiviral agents with antiviral strategies in existing studies ranging from short courses to indefinite duration. Monitoring for HBV reactivation after cessation of antivirals may mitigate adverse outcomes. Nonetheless, there are rare cases of significant recipient HBV reactivation and death in the setting of antiviral cessation or non-prescription.50,51 As such, an optimal duration of treatment is difficult to recommend, and should be individualised based on donor and recipient factors, in conjunction with a hepatologist or infectious diseases physician experienced in HBV; however, treatment is often indefinite.47 Yin S, Wu L, Zhang F, et al. Expanding the donor pool: Kidney transplantation from serum HBV DNA or HBeAg-positive donors to HBsAg-negative recipients. Liver Int. Nov 2023;43(11):2415-2424. doi:10.1111/liv.15703 48 Wang XD, Feng SJ, Liu JP, et al. Pre-transplant donor HBV DNA+ and male recipient are independent risk factors for treatment failure in HBsAg+ donors to HBsAg- kidney transplant recipients. BMC Infect Dis. Jan 9 2021;21(1):41. doi:10.1186/s12879-020-05704-1 ×46 Delman AM, Turner KM, Safdar K, et al. Expanding the Donor Pool: First Use of Hepatitis B Virus Nat Positive Solid Organ Allografts Into Seronegative Recipients. Ann Surg. Oct 1 2021;274(4):556-564. doi:10.1097/sla.0000000000005071 49 Carnemolla BT, Kutzler HL, Kuzaro HA, et al. Use of hepatitis B viremic donors in kidney transplant recipients: A single center experience. Transpl Infect Dis. Aug 2022;24(4):e13872. doi:10.1111/tid.13872 ×50 Tatar E, Turan MN, Firat O, et al. Use of kidney donors with hepatitis B, hepatitis C, or brain tumor: a single-center experience. Transplant Proc. Jul-Aug 2012;44(6):1601-3. doi:10.1016/j.transproceed.2012.04.028 51 Magiorkinis E, Paraskevis D, Pavlopoulou ID, et al. Renal transplantation from hepatitis B surface antigen (HBsAg)-positive donors to HBsAg-negative recipients: a case of post-transplant fulminant hepatitis associated with an extensively mutated hepatitis B virus strain and review of the current literature. Transplant Infectious Disease. 2013;15(4):393-399. doi:https://doi.org/10.1111/ tid.12094 ×
HBsAg-negative, HBV NAT-negative, HBcAb-positive donors
Interpretations include:
Past infection
― HBcAb of immunoglobulin M (IgM) class indicates a recent infection with HBV, while HBcAb of immunoglobulin G (IgG) class generally indicates a past infection
― HBsAb will typically be positive but may be lost in the case of longstanding past infection, or may not have appeared after recent acute infectionPersistent hepatocyte infection
― The liver is a reservoir for HBV, and HBcAb-positive donor hepatocytes contain HBV cccDNA, with reactivation possible at any time in liver recipients52,53False-positive test result.
Liver recipients
For donors who are HBcAb-positive/HBsAg-negative, transmission rates are higher for liver transplantation than for the transplantation of other solid organs – 34% to 86% without prophylaxis in liver recipients54,55 versus 0% to 5% for recipients of non-liver organs.56 Prophylaxis for recipients of livers from HBcAb-positive donors has been shown to be effective, although transmission of HBV has been reported in rare instances despite this, mostly in the setting of lamivudine usage rather than antivirals with a high barrier to resistance (entecavir and tenofovir).57,58,5954 Levitsky J, Doucette K, AST Infectious Diseases Community of Practice. Viral Hepatitis in Solid Organ Transplantation. Am J Transplant, 2013;13(suppl 4):147-168. 55 Nery JR, Nery-Avila C, Reddy KR, et al.. Use of liver grafts from donors positive for antihepatitis B-core antibody (anti-HBc) in the era of prophylaxis with hepatitis-B immunoglobulin and lamivudine. Transplantation, 2003;75(8):1179-86. ×56 Fabrizio F, Bunnapradist S, and Martin P. Transplanting kidneys from donors with prior hepatitis B infection: one response to the organ shortage. J Nephrol, 2002;15(6):605-13. ×57 Cholongitas E, Papatheodoridis GV, and Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol, 2010;52(2):272-9. 43 44. 58 Salvadori M, Rosso G, Carta P, et al. Donors positive for hepatitis B core antibodies in non-liver transplantations. Transplant Proc, 2011;43(1):277-9. 59 Dhillon GS, Levitt J, Mallifi H, et al. Impact of hepatitis B core antibody positive donors in lung and heart-lung transplantation: an analysis of the United Network For Organ Sharing Database. Transplantation, 2009;88(6):842-6. ×
While it is preferred that livers from HBcAb-positive donors be used for recipients with current or previous HBV infection, or recipients who have been successfully vaccinated, transplantation to naïve recipients can also be considered. Indefinite antiviral prophylaxis should be considered for all liver recipients given long-term risks of reactivation of infection within transplanted hepatocytes.
Non-liver recipients
For non-liver organs, the risk of transmission is negligible for recipients who are immune. Non-liver organs from donors who are HBcAb-positive and HBsAg-negative/NAT-negative may be used for HBV-naïve recipients after informed consent and with HBsAg and HBV DNA testing of the recipient. Short durations of antiviral prophylaxis (entecavir or tenofovir) for non-immune recipients may be considered.
Donors at increased risk of HBV
If the donor social or medical history is suggestive of increased risk of HBV infection (see Section 2.3.2), test results should be interpreted in the context of donor risk factors, particularly if NAT results are not available prior to transplantation. See Table 2.4 for the residual risks of an eclipse/window period HBV infection, by risk factor.
Table 2.5: Interpretation of results of HBV screening in organ donors and recommendations for utilisation
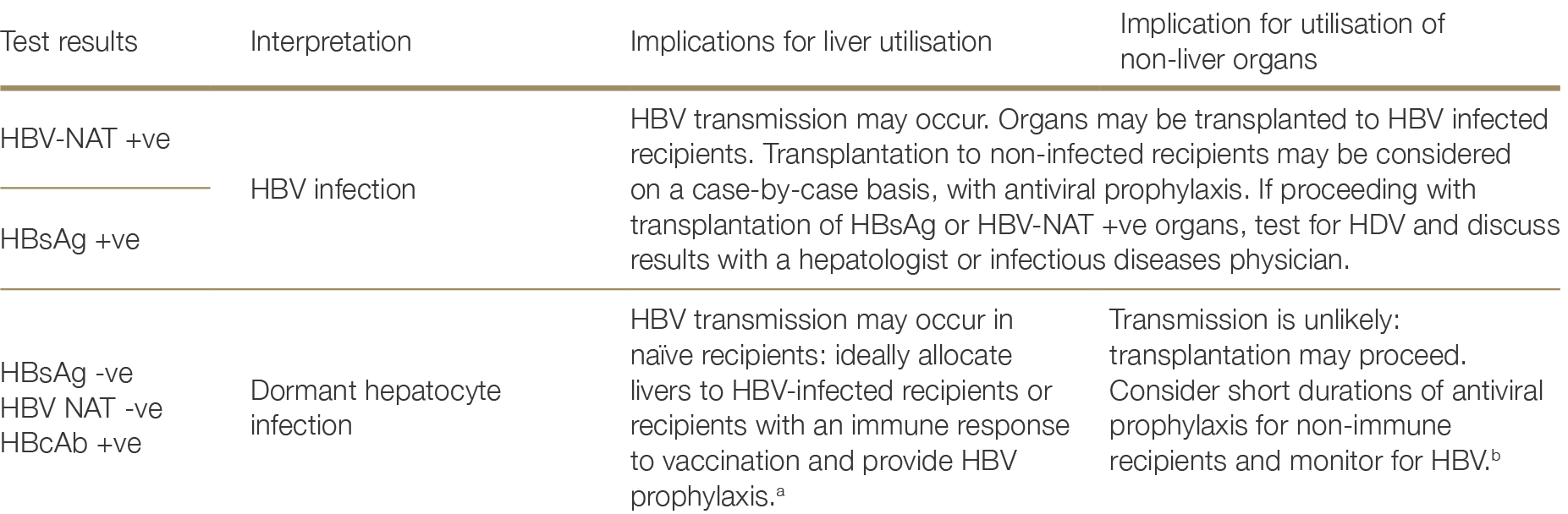
a Recipient management would typically involve life-long entecavir or tenofovir with HBsAg and HBV DNA monitoringa No reference text available.×
b For the non-liver recipient with HBsAb >100 IU/L no prophylaxis is required. For the non-liver recipient with HBsAb < 100 IU/L, consider short durations of entecavir or tenofovir. Non-liver recipients should be tested by HBsAg and HBV DNA to 12 months post-transplant.b No reference text available.×
Figure 2.2: Decision flow-chart for HBV testing and utilisation of HBV-positive donors
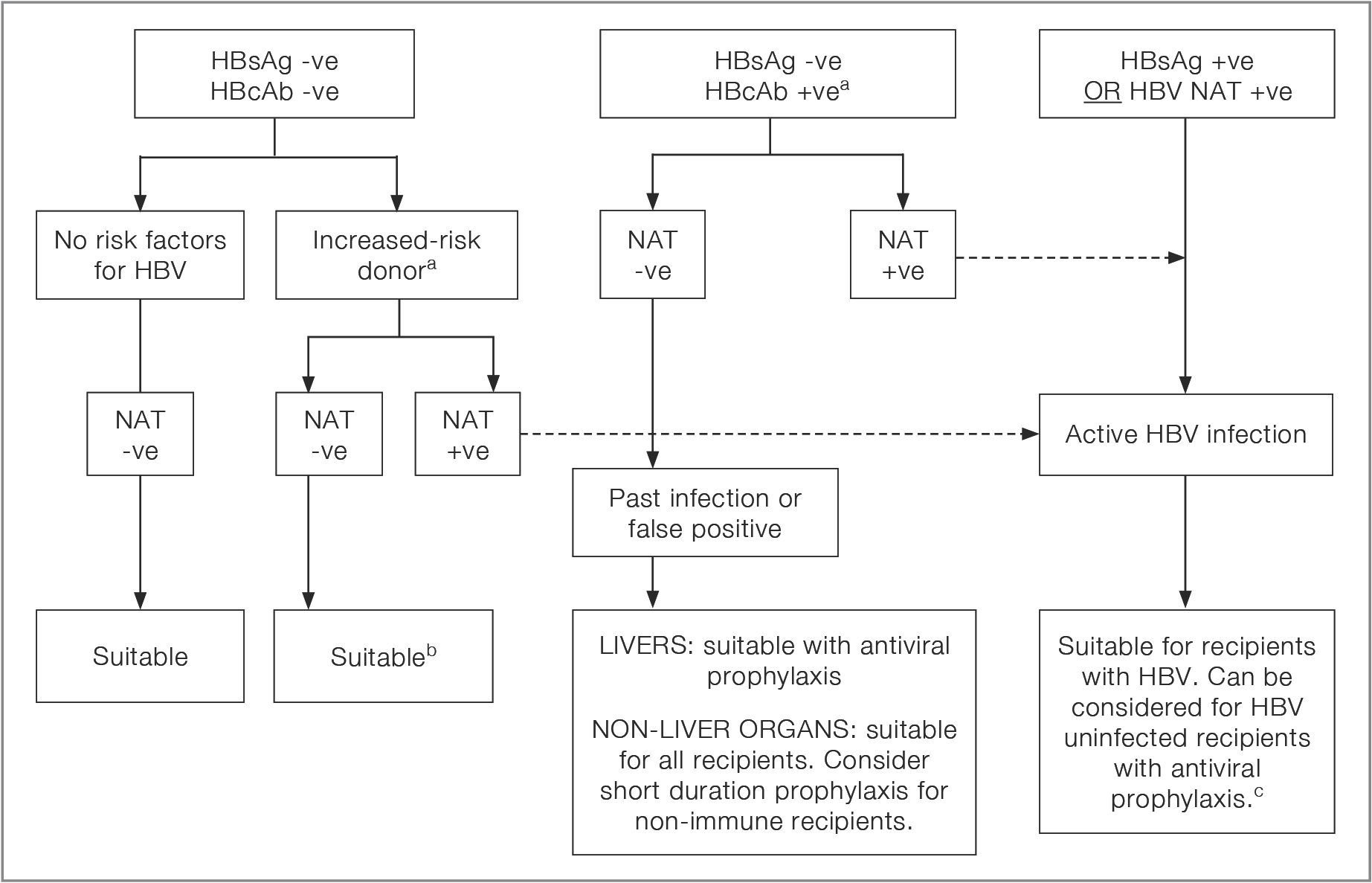
a If NAT result is not available, follow the pathway for a NAT +ve donor, taking into account the nature of donor risk factorsa No reference text available.×
b Consider the possibility of an eclipse period infectionb No reference text available.×
c The use of donors with active HBV for non-infected recipients should have specialist hepatology or infectious diseases input, recipient consent, and antiviral prophylaxis.c No reference text available.×
2.3.2.5 Hepatitis C virus
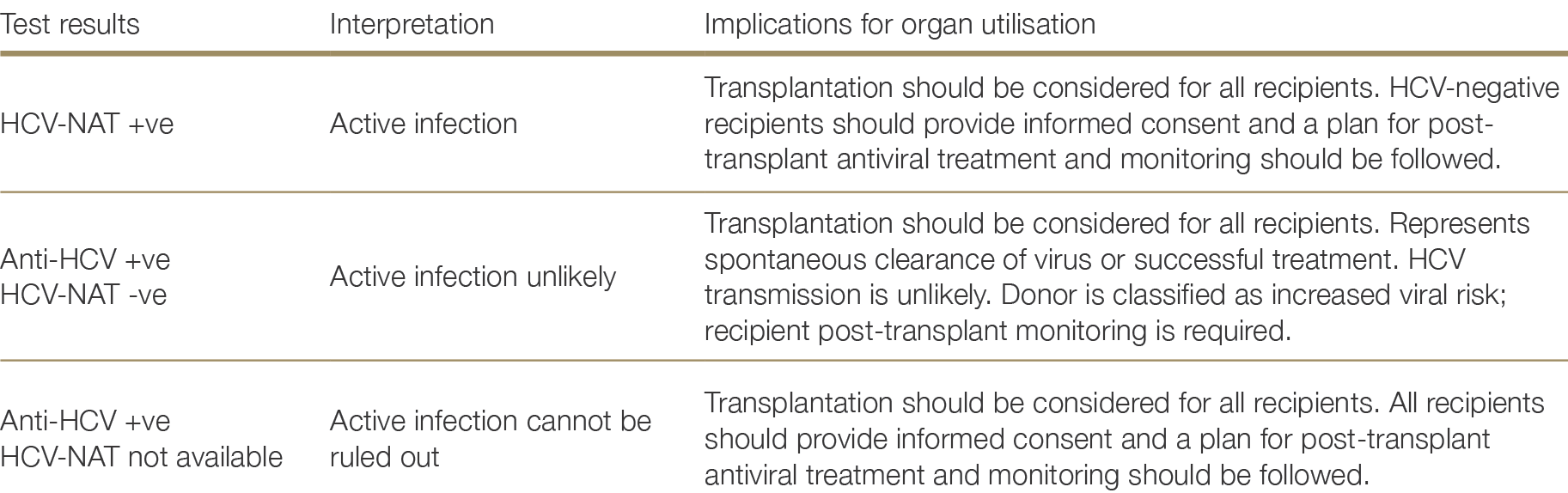
A positive HCV-NAT, with or without a positive anti-HCV, indicates active HCV infection. A positive anti-HCV with a negative HCV-NAT essentially excludes active HCV infection, given the low level of virus which can be detected with current RNA assays, excluding a short eclipse period risk of re-infection. Both anti-HCV and HCV-NAT are recommended for all donors. Figure 2.3 depicts the decision flow-chart for HCV testing, whilst Table 2.6 summarises the suggested organ utilisation and management implications.
Anti-HCV-positive, NAT negative donors
The risk of transmission from NAT-negative, anti-HCV-positive non-liver organs is very low.60,61,62 Previous HCV infection is, however, a risk factor for reinfection, and classifies a donor as one of increased viral risk.63 Should HCV transmission occur, HCV in the recipient is highly treatable. As such, routine use of anti-HCV positive, HCV NAT-negative non-liver donors can occur, with testing according to receipt of an increased viral risk organ.60 Vera ME, Volk ML, Ncube Z et al. Transplantation of hepatitis C virus (HCV) antibody positive, nucleic acide test negative donor kidneys to HCV negative patients frequently results in seroconversion by not HCV viraemia. Am J Transplant, 2018; 18 (2451- 2456). 61 Dao A, Cuffy M, Kaiser TE et al. Use of HCV Ab+/NAT- donors in HCV naïve renal transplant recipients to expand the kidney donor pool. Clin Transplant. 2019 Jul;33(7): e13598. 62 Franco A, Moreso F, Merino E et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: a prospective study. Transpl Int. 2019 Jul;32(7):710-716. ×63 Anesi JA, Goldberg DS. Maximizing Utilization of the Donor Pool by Appropriate Classification of Hepatitis C Antibody-Positive Donors. Am J Transplant. 2017 Nov;17(11):2757-2758. ×
Figure 2.3: Decision flow-chart for HCV testing and utilisation of HCV-positive donors
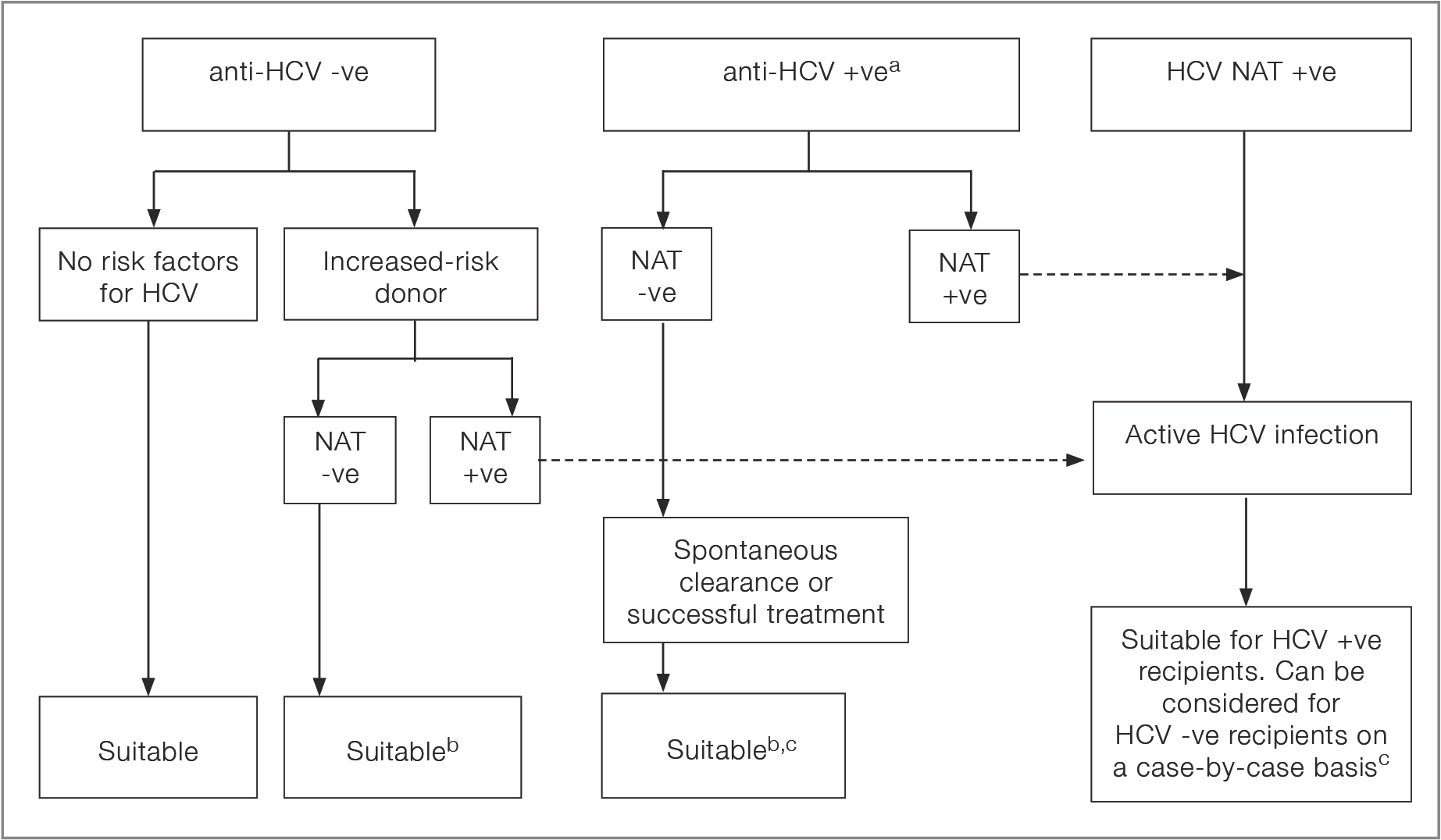
a If NAT result is not available, follow the pathway for a NAT +ve donor, taking into account the nature of donor risk factorsa No reference text available.×
b Consider the possibility of an eclipse period infectionb No reference text available.×
c Livers from donors who test HCV Ab pos NAT negative can transmit HCV, and from NAT positive donors would only be suitable in the absence of significant fibrosis/cirrhosis in the donorc No reference text available.×
Table 2.6: Interpretation of results of HCV screening in organ donors and recommendations for utilisation
HCV-NAT-positive donors (active infection)
The reported cure rate of HCV after transplantation after first-line direct acting antiviral therapy is high (>90%),64,65 including for liver transplantation, and receipt of HCV NAT positive organ does not appear to influence allograft outcomes.66,67 Therefore, organs from HCV NAT positive donors can routinely be considered for transplantation into HCV-negative recipients.65,67,68,69,70,71 Livers should only be transplanted in the absence of significant fibrosis, as per usual assessment. As transmission from HCV NAT positive donor organs is near universal, recipients are expected to require antiviral treatment, commenced early after transplantation (noting complications such as cholestatic hepatitis and glomerulonephritis occurred with delayed antiviral therapy). The potential risks, complications, and requirements for post- transplant antiviral therapy need to be discussed with potential recipients to ensure robust informed consent is obtained.64 Schlendorf KH, Zalawadiya S, Shah AS, et al. Expanding Heart Transplant in the Era of Direct-Acting Antiviral Therapy for Hepatitis. C. JAMA Cardiol. 2020 Feb 1;5(2):167-174. 65 Gordon CE, Adam GP, Jadoul M, Martin P, Balk EM. Kidney Transplantation From Hepatitis C Virus-Infected Donors to Uninfected Recipients: A Systematic Review for the KDIGO 2022 Hepatitis C Clinical Practice Guideline Update. Am J Kidney Dis. 2023 Oct;82(4):410-418. ×66 Schaubel DE, Tran AH, Abt PL, Potluri VS, Goldberg DS, Reese PP. Five-Year Allograft Survival for Recipients of Kidney Transplants From Hepatitis C Virus Infected vs Uninfected Deceased Donors in the Direct-Acting Antiviral Therapy Era. JAMA. 2022 Sep 20;328(11):1102-1104. 67 Woolley AE, Singh SK, Goldberg HJ et al. DONATE HCV Trial Team. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019 Apr 25;380(17):1606-1617. ×65 Gordon CE, Adam GP, Jadoul M, Martin P, Balk EM. Kidney Transplantation From Hepatitis C Virus-Infected Donors to Uninfected Recipients: A Systematic Review for the KDIGO 2022 Hepatitis C Clinical Practice Guideline Update. Am J Kidney Dis. 2023 Oct;82(4):410-418. 67 Woolley AE, Singh SK, Goldberg HJ et al. DONATE HCV Trial Team. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019 Apr 25;380(17):1606-1617. 68 Goldberg DS et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med, 2017; 376(24): 2394-2395. 69 Durand C et al. EXPANDER-1: Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients. Am J Transplant, 2017; 17(Suppl 3). 70 Saberi B et al. Utilization of hepatitis C virus RNA-positive donor liver for transplant to hepatitis C virus negative recipient. Liver Transpl, 2018; 24(1):140-143. 71 Aslam S, Grossi P, Schlendorf KH, Holm AM et al. Utilization of hepatitis C virus-infected organ donors in cardiothoracic transplantation: An ISHLT expert consensus statement. J Heart Lung Transplant. 2020 May;39(5):418-432. doi: 10.1016/j. healun.2020.03.004. Epub 2020 Mar 19. PMID: 32362393. ×
2.3.2.6 Herpes simplex virus
The overall seroprevalence of HSV-1 and HSV-2 in the Australian population is 76% and 12% respectively, although actual rates are highly variable by age group and according to risk factors for acquisition.72 In the absence of appropriate prophylaxis, life-threatening de novo infections have occurred in naïve recipients of organs from latently-infected donors,73,74 and due to reactivation in latently-infected recipients.75 Given high rates of donor and recipient exposure, routine prophylaxis seems a more efficient approach than donor and recipient HSV-1 and HSV-2 IgG testing. Routine HSV prophylaxis is supported by a number of guidelines.76 Where it is administered, CMV antiviral prophylaxis will also be effective against HSV. In the event that CMV prophylaxis is not given, acyclovir, famciclovir or valaciclovir would be the anti-HSV agents commonly utilised, usually recommended for at least one-month post-transplantation. Active infection in donors should also be considered where there are clinical features suggestive of HSV.72 Cunningham AL, Taylor R, Taylor J, et al. Prevalence of infection with herpes simplex virus types 1 and 2 in Australia: a nationwide population based survey. Sex Transm Infect, 2006; 82(2):164-8. ×73 Macesic N, Abbott IJ, Kaye M, et al. Herpes simplex virus-2 transmission following solid organ transplantation: Donor-derived infection and transplantation from prior organ recipients. Transpl Infect Dis, 2017; 19(5). 74 Setyapranata S, Holt SG, Wiggins KJ, et al. Renal allograft re-use and herpectic re-infection. Nephrology, 2015; 20 (suppl 1): 17-2. ×75 Shiley, K, Blumberg E. Herpes viruses in transplant recipients: HSV, VZV, Human Herpes viruses, and EBV. Infect Dis Clin N Am,2010; 24:373-393. ×76 Lee DH, Zuckerman RA; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep;33(9): e13526. ×
Recommendation
Organs can be accepted from donors with latent herpes family viral infections, and HSV screening is not required where antiviral prophylaxis is routinely administered. Organs from donors with acute herpes viraemia should only be considered with the administration of HSV-active antiviral treatment to the recipient.
2.3.2.7 Human herpesvirus-8 (Kaposi’s sarcoma-associated herpesvirus)
Human herpes virus-8 (HHV-8) is a rare but potentially fatal infection that can cause a spectrum of diseases in solid organ transplant recipients. Transmission occurs predominantly via saliva but also sexual, vertical and parenteral (including intravenous drug use). Following primary infection, HHV-8 may persist in a latent state with potential to reactivate and cause disease, particularly in immunosuppressed individuals. In solid organ transplant recipients, HHV-8 disease may result from donor-derived transmission, reactivation of latent infection in the recipient or de novo acquisition post-transplant.
Population level risk factors for HHV-8 infection
in North America and northern Europe to between 20-80% in the Mediterranean, parts of Africa and parts of China.
Studies in Australia have reported HHV8 seroprevalence of 5.8% in blood donors,78 compared with 18% in men who have sex with men (MSM).79 Risk factors for HHV-8 infection in the Australian population hence include residence in an HHV-8 endemic region, and risk behaviors for other blood borne viruses (hepatitis B, hepatitis C and human immunodeficiency virus), such as MSM, commercial sex work, incarceration or a child with a mother with HHV-8 infection.80,8178 Speicher, D. J., Fryk, J. J., Kashchuk, V., Faddy, H. M., & Johnson, N. W. (2022). Human Herpesvirus 8 in Australia: DNAemia and Cumulative Exposure in Blood Donors. Viruses, 14(10), 2185. https://doi.org/10.3390/v14102185 ×79 Grulich, A. E., Cunningham, P., Munier, M. L., Prestage, G., Amin, J., Ringland, C., Whitby, D., Kippax, S., Kaldor, J. M., & Rawlinson, W. (2005). Sexual behaviour and human herpesvirus 8 infection in homosexual men in Australia. Sexual health, 2(1), 13–18. https://doi.org/10.1071/sh04029 ×80 Dollard, S. C., Annambhotla, P., Wong, P., Meneses, K., Amin, M. M., La Hoz, R. M., Lease, E. D., Budev, M., Arrossi, A. V., Basavaraju, S. V., & Thomas, C. P. (2021). Donor-derived human herpesvirus 8 and development of Kaposi sarcoma among 6 recipients of organs from donors with high-risk sexual and substance use behavior. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 21(2), 681–688. https://doi. org/10.1111/ajt.16181 81 Nambiar, P., Liang, T., Labo, N., Hand, J., Blumberg, E. A., Rana, M. M., Florman, S., Haydel, B., Morris, M. I., Schaenman, J., Rodrigues, M. M. S., Werbel, W. A., Bowring, M. G., Friedman-Moraco, R. J., Stock, P., Stosor, V., Mehta, S., Gilbert, A. J., Elias, N., Mehta, S. A., … Durand, C. M. (2025). Kaposi Sarcoma-Associated Herpesvirus Risk and Disease in Kidney Donors and Transplant Recipients with HIV in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaf229. Advance online publication. https://doi.org/10.1093/cid/ciaf229 ×
Spectrum of HHV-8 diseases in solid organ transplant recipients
While most immunocompetent hosts are asymptomatic, the spectrum of disease manifestations associated with HHV-8 infection includes a range of malignant, lymphoproliferative and inflammatory conditions, which can co-exist. Diseases include:
Visceral and cutaneous Kaposi sarcoma (KS) Malignancy of endothelial cells (blood vessels and lymphatics) with characteristics elongated ‘spindle’ cells. Visceral KS may occur without characteristic skin lesions post-transplant, affecting lymph nodes and/or organs, including the allograft.
Multi-centric Castleman’s disease (MCD) Lymphoproliferative disorder with multiple enlarged lymph nodes (and characteristic biopsy findings), associated with systemic symptoms.
Primary effusion lymphoma (PEL) and other hematologic malignancies PEL is a rare diffuse large B cell lymphoma which accumulates in body cavities. Other HHV8 forms of PTLD have also been described.
Kaposi Sarcoma Inflammatory Cytokine Syndrome (KICS) Non-malignant inflammatory condition associated with high level HHV-8 viraemia. Relatively recently described,82 occurring most commonly post-transplant among liver recipients, due to donor derived transmission (DDT).
Hemophagocytic lymphohistiocytosis (HLH)82,83,84,85,86 Inflammatory condition which can be triggered by HHV8 (often co-occurring with other HHV8 diseases).82 Polizzotto, M. N., Uldrick, T. S., Wyvill, K. M., Aleman, K., Marshall, V., Wang, V., Whitby, D., Pittaluga, S., Jaffe, E. S., Millo, C., Tosato, G., Little, R. F., Steinberg, S. M., Sereti, I., & Yarchoan, R. (2016). Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 62(6), 730–738. https://doi.org/10.1093/cid/civ996 83 Razonable R. R. (2013). Human herpesviruses 6, 7 and 8 in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 13 Suppl 3, 67–78. https://doi.org/10.1111/ajt.12008 84 Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV related diseases in posttransplant patients. Blood 2012;120:4150—9. https://doi.org/10.1182/blood-2012-04-421412. 85 Luppi M, Cona A, Barozzi P, Riva G, Mularoni A. Differential diagnosis between kaposi sarcoma-associated herpesvirus cytokine syndrome and hemophagocytic lymphohistiocytosis. Infection 2024;52:1643—4. https:// doi.org/10.1007/s15010-024-02252-7 86 Bonazzetti, C., Rinaldi, M., Giovagnorio, F., İrkören, P., Casarini, M., Cascavilla, A., Morelli, M. C., Vizioli, L., Pianta, P., Comai, G., Ravaioli, M., Potena, L., Gabrielli, L., Lazzarotto, T., Alvaro, N., Viale, P., & Giannella, M. (2025). HHV8-related diseases in solid organ transplantation: a case series and systematic literature review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, S1198-743X(25)00360-X. Advance online publication. https://doi.org/10.1016/j.cmi.2025.07.019 ×
The 90-day mortality for HHV-8 related diseases post-transplant (excluding cutaneous KS) is 29%,86 with rates more than 50% reported in the setting of DDT.87 The highest risk of DDT is from infected donors to uninfected recipients. Lymphoid rich organs such as liver and lungs appear to be associated with the greatest risk of HHV-8 DDT,88,89 although cases have been reported across the spectrum of transplanted organs.8686 Bonazzetti, C., Rinaldi, M., Giovagnorio, F., İrkören, P., Casarini, M., Cascavilla, A., Morelli, M. C., Vizioli, L., Pianta, P., Comai, G., Ravaioli, M., Potena, L., Gabrielli, L., Lazzarotto, T., Alvaro, N., Viale, P., & Giannella, M. (2025). HHV8-related diseases in solid organ transplantation: a case series and systematic literature review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, S1198-743X(25)00360-X. Advance online publication. https://doi.org/10.1016/j.cmi.2025.07.019 ×87 UK Government Department of Health and Social Care. Independent Report: SaBTO virology subcommittee recommendations on KSHV: executive summary. March 2025. Accessed 24th October 2025. Available here: SaBTO virology subcommittee recommendations on KSHV: executive summary – GOV.UK ×86 Bonazzetti, C., Rinaldi, M., Giovagnorio, F., İrkören, P., Casarini, M., Cascavilla, A., Morelli, M. C., Vizioli, L., Pianta, P., Comai, G., Ravaioli, M., Potena, L., Gabrielli, L., Lazzarotto, T., Alvaro, N., Viale, P., & Giannella, M. (2025). HHV8-related diseases in solid organ transplantation: a case series and systematic literature review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, S1198-743X(25)00360-X. Advance online publication. https://doi.org/10.1016/j.cmi.2025.07.019 ×
All solid organ transplant recipients, but especially those at increased risk due to donor or recipient risk factors, should be monitored closely for the development of disease manifestations consistent with a diagnosis of HHV-8 and investigated early, particularly during the first 12 months post-transplant when the risk of HHV-8- related disease is greatest.80,81,86,88,89 Transplant clinicians should be aware of these rare diseases and seek expert advice on diagnosis and management, which is rapidly evolving. HHV-8 DNA monitoring should only be performed under guidance from a transplant infectious diseases specialist.80 Dollard, S. C., Annambhotla, P., Wong, P., Meneses, K., Amin, M. M., La Hoz, R. M., Lease, E. D., Budev, M., Arrossi, A. V., Basavaraju, S. V., & Thomas, C. P. (2021). Donor-derived human herpesvirus 8 and development of Kaposi sarcoma among 6 recipients of organs from donors with high-risk sexual and substance use behavior. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 21(2), 681–688. https://doi. org/10.1111/ajt.16181 81 Nambiar, P., Liang, T., Labo, N., Hand, J., Blumberg, E. A., Rana, M. M., Florman, S., Haydel, B., Morris, M. I., Schaenman, J., Rodrigues, M. M. S., Werbel, W. A., Bowring, M. G., Friedman-Moraco, R. J., Stock, P., Stosor, V., Mehta, S., Gilbert, A. J., Elias, N., Mehta, S. A., … Durand, C. M. (2025). Kaposi Sarcoma-Associated Herpesvirus Risk and Disease in Kidney Donors and Transplant Recipients with HIV in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaf229. Advance online publication. https://doi.org/10.1093/cid/ciaf229 86 Bonazzetti, C., Rinaldi, M., Giovagnorio, F., İrkören, P., Casarini, M., Cascavilla, A., Morelli, M. C., Vizioli, L., Pianta, P., Comai, G., Ravaioli, M., Potena, L., Gabrielli, L., Lazzarotto, T., Alvaro, N., Viale, P., & Giannella, M. (2025). HHV8-related diseases in solid organ transplantation: a case series and systematic literature review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, S1198-743X(25)00360-X. Advance online publication. https://doi.org/10.1016/j.cmi.2025.07.019 88 Mularoni, A., Cona, A., Bulati, M., Busà, R., Miele, M., Timoneri, F., Di Bella, M., Castelbuono, S., Barbera, F., Di Carlo, D., Volpe, L., Gallo, A., Maria de Luca, A., Coniglione, G., Todaro, F., Barozzi, P., Riva, G., Pietrosi, G., Gruttadauria, S., Bertani, A., … Luppi, M. (2025). Serologic screening and molecular surveillance of Kaposi sarcoma herpesvirus/human herpesvirus-8 infections for early recognition and effective treatment of Kaposi sarcoma herpesvirus-associated inflammatory cytokine syndrome in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 25(5), 1070–1085. https://doi.org/10.1016/j.ajt.2024.11.013 89 Cahoon, E. K., Linet, M. S., Clarke, C. A., Pawlish, K. S., Engels, E. A., & Pfeiffer, R. M. (2018). Risk of Kaposi sarcoma after solid organ transplantation in the United States. International journal of cancer, 143(11), 2741–2748. https://doi.org/10.1002/ijc.31735 ×
Mitigating strategies for donor-derived transmission
HHV-8 donor screening results to influence donor suitability
Currently there is insufficient evidence that donor HHV-8 results should influence donor suitability assessment. Internationally where donor HHV-8 screening is undertaken, results do not determine donor suitability.
HHV-8 donor screening to guide recipient monitoring and early intervention
Internationally, some centers (33% in one survey90), mostly in HHV-8 endemic regions, use donor screening to identify recipients at increased risk of donor-derived transmission of HHV-8 who may benefit from surveillance post-transplant. These screening programs have diverse methods and contrary results. In the United Kingdom (UK), a non-endemic region, nationwide donor screening was implemented in 2023, using HHV-8 serology followed by confirmation with HHV-8 DNA testing. Preliminary data from the UK found confirmed HHV-8 DDT events only occurred in the setting of donors with a positive DNAemia (10/3269 screening donors), with no instances of transmission from donors who were HHV-8 seropositive but DNA negative (unpublished data). In contrast, review of a universal serological screening program at an Italian center between 2011–2023 identified a seroprevalence of 3.3% among donors and 8.4% in recipients. In 49 instances of serological mismatch (D+/R-), 22 recipients (45%) experienced DDT and 13 recipients (26.5%) developed HHV-8 related disease complications.8890 Mularoni, A., Mikulska, M., Giannella, M., Adamoli, L., Slavin, M., Van Delden, C., Garcia, J. M. A., Cervera, C., & Grossi, P. A. (2021). International survey of human herpes virus 8 screening and management in solid organ transplantation. Transplant infectious disease: an official journal of the Transplantation Society, 23(5), e13698. https://doi.org/10.1111/tid.13698 ×88 Mularoni, A., Cona, A., Bulati, M., Busà, R., Miele, M., Timoneri, F., Di Bella, M., Castelbuono, S., Barbera, F., Di Carlo, D., Volpe, L., Gallo, A., Maria de Luca, A., Coniglione, G., Todaro, F., Barozzi, P., Riva, G., Pietrosi, G., Gruttadauria, S., Bertani, A., … Luppi, M. (2025). Serologic screening and molecular surveillance of Kaposi sarcoma herpesvirus/human herpesvirus-8 infections for early recognition and effective treatment of Kaposi sarcoma herpesvirus-associated inflammatory cytokine syndrome in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 25(5), 1070–1085. https://doi.org/10.1016/j.ajt.2024.11.013 ×
Protocols for management after identifying at-risk recipients are also limited and inconsistent. Antivirals have limited efficacy for HHV-8 and there is no role for primary prophylaxis. Pre-emptive monitoring of D+/R- using HHV-8 NAT has been described, with modulation of immunosuppression in response to detectable DNAemia as one possible strategy. However, not all patients with DDT will develop HHV-8 disease, and any benefits from pre-emptive immunosuppressive reduction may be offset by increased rejection rates. Close monitoring for disease and early initiation of B cell depletion with rituximab upon diagnosis of KICS has been described in an Italian cohort88 associated with favorable outcomes. However, standardized screening tests, thresholds for intervention and treatment efficacy remain undefined.88 Mularoni, A., Cona, A., Bulati, M., Busà, R., Miele, M., Timoneri, F., Di Bella, M., Castelbuono, S., Barbera, F., Di Carlo, D., Volpe, L., Gallo, A., Maria de Luca, A., Coniglione, G., Todaro, F., Barozzi, P., Riva, G., Pietrosi, G., Gruttadauria, S., Bertani, A., … Luppi, M. (2025). Serologic screening and molecular surveillance of Kaposi sarcoma herpesvirus/human herpesvirus-8 infections for early recognition and effective treatment of Kaposi sarcoma herpesvirus-associated inflammatory cytokine syndrome in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 25(5), 1070–1085. https://doi.org/10.1016/j.ajt.2024.11.013 ×
Use of HHV-8 donor screening in Australia and New Zealand
Serological tests for HHV-8 are not currently available. HHV-8 NAT assays are available in some jurisdictions, however test performance for donor screening is uncertain and donor derived HHV-8 infection has occurred with negative donor NAT results.
Where a case of HHV-8 DDT has been confirmed, the recipients of other organs from the same donor shouldbe notified with, at a minimum, clinical monitoring for HHV-8 disease, and optional HHV-8 PCR testing to assist risk stratification.
Recommendation
Routine donor screening for HHV-8 is not recommended. Potential donors should not be excluded from donation based on perceived risk of HHV-8.Solid organ transplant recipients should be tested for HHV-8 in the context of a compatible illness.If a solid organ transplant recipient is found to have HHV-8 related disease, expert consultation should be soughtfor appropriate management. The possibility of donor-derived infection must be considered and reported throughthe appropriate channels, to facilitate monitoring of other recipients, as necessary, in accordance with expertguidance.
2.3.2.8 Human immunodeficiency virus
Screening for HIV should be performed using both NAT and a fourth-generation antigen/antibody combination immunoassay. These fourth-generation antigen/antibody combination immunoassays identify antibodies against both HIV-1 and HIV-2 as well as the presence of p24 antigen. If an initial test is positive, this result should be confirmed with subsequent testing according to jurisdictional policies, which may include separate antibody and p24 antigen assays, commercial western blotting assays, and/or nucleic acid tests.
The use of organs from donors with HIV for recipients with HIV (D+/R+) is supported by increasing evidence from both high and low HIV prevalence settings. Three studies of kidney HIV D+/R+ transplantation show favourable outcomes compared to HIV D-/R+ transplants, although one study demonstrated a trend towards increased rejection among HIV D+ kidney transplant recipients, which warrants additional investigation.91,92,93 Notably, the risk of rejection was half as common in those receiving lymphocyte depleting induction, indicating that opportunities may exist to mitigate this risk. One cohort of D+/R+ liver transplants showed similar overall and graft survival, but increased opportunistic infections, mostly driven by CMV infection (among high risk CMV mismatch pairs, numerically higher in the HIV D+/R+ group) and HHV-8-related malignancies (not statistically significant).94 Experience with other solid organs is more limited, but outcomes are not expected to be significantly different.95 HIV superinfection with a drug-resistant strain is a theoretical concern, and while donor HIV is detectable in a proportion of recipients early after transplantation, the clinical relevance remains unclear.94,96,9791 Muller E, Barday Z, Mendelson M, Kahn D. HIV-Positive–to–HIV-Positive Kidney Transplantation — Results at 3 to 5 Years. New England Journal of Medicine. 2015;372(7):613-620. doi:doi:10.1056/NEJMoa1408896 92 Durand CM, Zhang W, Brown DM, et al. A prospective multicenter pilot study of HIV-positive deceased donor to HIV-positive recipient kidney transplantation: HOPE in action. Am J Transplant. May 2021;21(5):1754-1764. doi:10.1111/ajt.16205 93 Durand CM, Massie A, Florman S, et al. Safety of Kidney Transplantation from Donors with HIV. New England Journal of Medicine. 2024;391(15):1390-1401. doi:doi:10.1056/NEJMoa2403733 ×94 Durand CM, Florman S, Motter JD, et al. HOPE in action: A prospective multicenter pilot study of liver transplantation from donors with HIV to recipients with HIV. Am J Transplant. Mar 2022;22(3):853-864. doi:10.1111/ajt.16886 ×95 Hemmige V, Saeed O, Puius YA, et al. HIV D+/R+ heart/kidney transplantation: First case report. The Journal of Heart and Lung Transplantation. 2023;42(3):406-408. doi:10.1016/j.healun.2022.11.004 ×94 Durand CM, Florman S, Motter JD, et al. HOPE in action: A prospective multicenter pilot study of liver transplantation from donors with HIV to recipients with HIV. Am J Transplant. Mar 2022;22(3):853-864. doi:10.1111/ajt.16886 96 Bonny TS, Kirby C, Martens C, et al. Outcomes of donor-derived superinfection screening in HIV-positive to HIV-positive kidney and liver transplantation: a multicentre, prospective, observational study. Lancet HIV. Sep 2020;7(9):e611-e619. doi:10.1016/ s2352-3018(20)30200-9 97 Selhorst P, Combrinck CE, Manning K, et al. Longer-Term Outcomes of HIV-Positive-to-HIV-Positive Renal Transplantation. N Engl J Med. Oct 3 2019;381(14):1387-1389. doi:10.1056/NEJMc1903013 ×
The number of transplant recipients with HIV internationally has been increasing, with good outcomes.98,99,100,101 However, people with HIV face additional barriers to waitlisting and transplantation.102,103,104 Data from the US indicate that a HIV D+/R+ strategy can provide a pathway to enhance access to transplantation for people with HIV and potentially higher quality organs, which may outweigh any potential additional risks.105 Moreover, it may enable utilisation of organs from donors with false-positive HIV screening tests, that would otherwise not be transplanted.106 In Australia, the incidence of donor referrals with HIV is also small , but successful HIV D+/ R+ kidney transplantation has been performed locally.107 An infectious diseases specialist with expertise in HIV and transplant infectious diseases should be involved in these cases, and specific informed consent should be obtained from the recipient. Donors with HIV must have an HIV infection that can effectively be treated using antiretroviral therapy that accounts for both efficacy and potential drug interactions in a post-transplant setting.98 McMullen L, Drak D, Basu G, et al. Kidney transplantation in people living with human immunodeficiency virus: An overview of the Australian experience. Nephrology (Carlton). Jan 2024;29(1):34-38. doi:10.1111/nep.14229 99 Waller KMJ, De La Mata NL, Hedley JA, et al. New blood-borne virus infections among organ transplant recipients: An Australian data-linked cohort study examining donor transmissions and other HIV, hepatitis C and hepatitis B notifications, 2000-2015. Transplant Infectious Disease. 2020;22(6):e13437. doi:https://doi.org/10.1111/tid.13437 100 Griffin DWJ, Kotecha S, Basu G, et al. Human immunodeficiency virus and solid organ transplantation: a 15-year retrospective audit at a tertiary Australian transplant centre. Intern Med J. Oct 2022;52(10):1780-1790. doi:10.1111/imj.15423 101 Zarinsefat A, Gulati A, Shui A, et al. Long-term Outcomes Following Kidney and Liver Transplant in Recipients With HIV. JAMA Surgery. 2022;157(3):240-247. doi:10.1001/jamasurg.2021.6798 ×102 Adekunle RO, Mehta AK, Wang Z, Patzer RE, Zhang R. Early steps to kidney transplantation among persons with HIV and end-stage renal disease in ESRD network 6. Transpl Infect Dis. Feb 2022;24(1):e13767. doi:10.1111/tid.13767 103 Locke JE, Mehta S, Sawinski D, et al. Access to Kidney Transplantation among HIV-Infected Waitlist Candidates. Clinical Journal of the American Society of Nephrology. 2017;12(3):467-475. doi:10.2215/cjn.07460716 104 Lee DH, Boyle SM, Malat GE, et al. Barriers to listing for HIV-infected patients being evaluated for kidney transplantation. Transpl Infect Dis. Dec 2017;19(6)doi:10.1111/tid.12777 ×105 Motter JD, Hussain S, Brown DM, et al. Wait Time Advantage for Transplant Candidates With HIV Who Accept Kidneys From Donors With HIV Under the HOPE Act. Transplantation. Mar 1 2024;108(3):759-767. doi:10.1097/tp.0000000000004857 ×106 Durand CM, Halpern SE, Bowring MG, et al. Organs from deceased donors with false-positive HIV screening tests: An unexpected benefit of the HOPE act. Am J Transplant. Oct 2018;18(10):2579-2586. doi:10.1111/ajt.14993 ×107 Holman J, Robinson A. 311.1: Pushing the boundaries - Leading change in Australian organ donation and transplantation: First HIV positive donor to HIV positive recipient. Transplantation. 2023;107(10S1):71. doi:10.1097/01.tp.0000993396.64427.a9 ×
Organ transplantation from donors with HIV into recipients without HIV is not routine practice, due to anticipated HIV transmission and limited evidence base. As such, there is insufficient published evidence to support the safety or clearly define the risks of this approach, even in exceptional cases. Should circumstances arise where a donor with HIV is appropriate to consider for a recipient without HIV (i.e. where the urgency and availability of transplantation is thought to outweigh the additional recipient risks of HIV transmission108), careful consideration would be required regarding consent processes, local approvals, and jurisdictional legal implications. Consultation of an infectious diseases specialist with expertise in HIV would be mandatory.108 Etheredge HR, Fabian J, Duncan M, Conradie F, Tiemessen C, Botha J. Needs must: living donor liver transplantation from an HIV-positive mother to her HIV-negative child in Johannesburg, South Africa. J Med Ethics. May 2019;45(5):287-290. doi:10.1136/ medethics-2018-105216 ×
Recommendation
All donors should be screened for HIV using an HIV Ag/Ab combination assay and HIV-NAT. Use of organs from selected donors with an antibody and/or NAT-positive for HIV should be considered for waitlisted recipients with HIV, in consultation with a HIV specialist. Donation of organs from donors with HIV for recipients without HIV is not recommended; should exceptional circumstances arise, consultation with a HIV specialist, informed consent and careful consideration of risks would be required.
2.3.2.9 Human T-lymphotropic virus-1
Human T-lymphotropic virus-1 (HTLV-1) is an oncogenic retrovirus that is transmitted predominantly via breast-feeding, sexual intercourse or blood transfusion. While infection is usually asymptomatic, about 2-5% of infected individuals will subsequently develop acute T-cell leukemia/lymphoma (ATL), and a smaller proportion (0.25-4%) will develop HTLV-1 associated myelopathy (HAM).109 Human T-lymphotropic virus-1 is not a ubiquitous virus; instead, it is understood to be distributed throughout the world in clusters of high endemicity. A high prevalence of HTLV-1 is found in sub-populations of southwestern Japan, sub-Saharan Africa, the Caribbean, parts of South America, parts of the Middle East, and among Aboriginal people of central Australia.110 However, large global regions have not been investigated for the prevalence of HTLV-1, and its distribution remains unknown in much of the world. Similarly, outside of central Australia, little is known about the epidemiology of HTLV-1 in Australia and New Zealand. Studies conducted among mostly non-Aboriginal and Torres Strait Islander blood donors living in Australian cities have reported prevalence ranging from 0.001 to 0.032%.110 It is important to note that HTLV screening assays do not distinguish between HTLV-1 and HTLV-2 infection, however HTLV-2 has not been convincingly associated with human disease.109 Gonçalves DU, Proietti FA, Ribas JGR, et al. Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases. Clin Microbiol Rev, 2010;23(3):577–589. ×110 Gessain A and Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 infection. Front Microbiol, 2012; 3:388 ×110 Gessain A and Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 infection. Front Microbiol, 2012; 3:388 ×
To date, there have been 16 cases published worldwide of HTLV-associated disease following donor-derived transmission: 14 cases of HAM and 2 cases of cutaneous ATL, with onset of symptoms between 8 months and 4 years post-transplant.111,112,113,114 A recent case series from Japan reported a rate of seroconversion of 87% for HTLV-1 negative recipients receiving a kidney transplant from an HTLV-1 positive donor, and a rate of HTLV-associated disease of 40% following donor-derived transmission (median incubation period 3.8 years).114 Given the morbidity and mortality risk associated with HAM and ATL, utilisation of donors confirmed positive for HTLV1/2 is not recommended.111 Ramanan P et al. Donor-transmitted HTLV-1-Associated Myelopathy in a kidney transplant recipient-case report and literature review. Am J Transplant, 2014;14:2417. 112 Armstrong MJ, Corbett C, Rowe IA et al. HTLV-1 in solid organ transplantation: current challenges and future management strategies. Transplantation, 2012; 94(11):1075-1084. 113 Taylor GP. Human T-lymphotropic virus type 1 infection and solid organ transplantation. Rev Med Virol, 2018; 28: e1970. 114 Yamauchi J, Yamano Y and Yuzawa K. Risk of Human T-cell Leukemia virus type 1 infection in kidney transplantation. N Eng J Med, 2019; 380 (3):296-298. ×114 Yamauchi J, Yamano Y and Yuzawa K. Risk of Human T-cell Leukemia virus type 1 infection in kidney transplantation. N Eng J Med, 2019; 380 (3):296-298. ×
The HTLV-1/2 positivity rate among Australian organ donors is <0.1%, and to date there have been no reported cases of HTLV-1 transmission by organ donation in Australia or New Zealand. Nonetheless, universal screening of donors for HTLV-1/2 remains recommended at this time given the limitations of our understanding of HTLV-1 epidemiology, the practical challenges of targeted screening (i.e. accurately identifying individuals at high risk), the current lack of therapy for HTLV-1, and the severity of outcomes in the extremely rare event of donor-derived disease. In addition, HTLV-1/2 screening is currently an absolute requirement for eye and tissue donation (with the exception of cornea-only donation). The potential for false positive test results for HTLV-1/2 should be noted, based on international experience.115115 Kaul DR et al. Donor screening for human T-cell lymphotropic virus ½: changing paradigms for changing testing capacity. Am J Transplant, 2010; 10(2): 207-213. ×
Recommendation
HTLV-1/2 screening using serology is recommended for all organ donors, with prospective results preferable where possible. Where a serological screening test is reactive, confirmatory testing should be undertaken and donation should not proceed if HTLV-1/2 infection is confirmed. In the event of reactive screening antibody results which cannot be confirmed by subsequent testing in a timely manner, it is suggested to discuss with an infectious diseases physician or microbiologist the likelihood that results predict genuine HTLV infection, which is influenced by the strength of the test (e.g. signal to cut-off ratio) and the pre-test probability of infection. If a donor is retrospectively confirmed to be infected with HTLV-1 and organs are transplanted, monitoring of recipients for both infection and disease development is recommended.
2.3.2.10 Seasonal influenza
During each annual influenza season (June, July, August) approximately 5-10% of the population is affected.116,117 During this period, a potential lung donor has about a 1-2% chance of transmitting influenza, based on 10% of the population being affected and given that influenza virus can be recovered from respiratory secretions of infected persons for approximately one week.118116 Sullivan SG, Raupach J, Franklin LJ, et al. A brief overview of influenza surveillance systems in Australia, 2015. Commun Dis Intell Q Rep, 2016; 40(3):E351-E355. 117 Newall AT, Wood JG and Macintyre CR. Influenza-related hospitalisation and death in Australians aged 50 years and older. Vaccine, 2008; 26(17):2135-41. ×118 Meylan PR, Aubert JD and Kaiser L. Influenza transmission to recipient through lung transplantation. Transplant Infect Dis, 2007;9(1):55-7. ×
In general, non-lung organs from donors with influenza infection can be safely used. As persons infected with influenza viruses generally do not have virus in non-lung tissues, the risk of transmitting infection to recipients of solid organs other than lungs is low.119.119 No reference text available.×
Evaluation of potential lung donors for influenza-like symptoms or respiratory tract infection is essential to avoid life-threatening infection in the recipient in the early post-transplant period.120 In the event of donor-derived influenza transmission, however, successful antiviral treatment is possible.118120 Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant, 2005;5(8):2031-6. ×118 Meylan PR, Aubert JD and Kaiser L. Influenza transmission to recipient through lung transplantation. Transplant Infect Dis, 2007;9(1):55-7. ×
In the event of circulating influenza strains with antiviral resistance, influenza A subtyping may inform treatment options.
Recommendation
If influenza-like illness in the donor is suspected, influenza-specific NAT should be performed (although it is not essential to wait for the result before proceeding with transplantation). The presence of influenza is not a contraindication to the transplantation of non-lung organs. Utilisation of lungs should be considered on a case- by case basis. Post-transplant influenza treatment for 5–10 days is suggested for all recipients of organs from a donor infected with influenza.
2.3.2.11 West Nile virus
West Nile virus (WNV) is a mosquito borne virus commonly found in Africa, parts of Europe, the Middle East, North America and West Asia. West Nile virus infection is asymptomatic or associated with only mild-flu-like symptoms in the vast majority of cases (>99%). However, in some cases – and particularly among immunosuppressed persons – WNV may cause severe neuro-invasive disease, including meningitis, encephalitis and acute flaccid paralysis.121 Multiple cases of WNV transmission from organ donors to recipients have been reported, with a high rate of adverse outcomes.121,122 Compared to a mortality rate of 4% among symptomatic WNV cases in the general population, the mortality rate among transplant recipients with symptomatic WNV is approximately 25%.123121 https://www.who.int/news-room/fact-sheets/detail/west-nile-virus ×121 https://www.who.int/news-room/fact-sheets/detail/west-nile-virus 122 SaBTO position statement: West Nile virus and solid organ transplantation. Advisory Committee on the safety of blood, tissues and organs (SaBTO), 2013. (https://www.gov.uk/government/publications/west-nile-virus-and-solid-organ-transplantation-sabto- statement) ×123 Yango AF, Fischbach BV, Levey M, et sl. West Nile virus infection in kidney and pancreas transplant recipients in the Dallas-Fort Worth Metroplex during the 2012 Texas epidemic. Transplantation, 2014; 97(9):953-7. ×
Suitable vectors for WNV have not been shown to exist in New Zealand, and to date there have been no notified cases of WNV, including cases acquired abroad. In Australia, the Kunjin lineage of WNV is endemic to tropical northern Australia, although notifications of WNV or Kunjin virus infection are rare – on average <2 per year – with some of these cases acquired in endemic countries.124124 Knope KE, Muller M, Kurucz, et al. Arboviral diseases and malaria in Australia 2013-14: annual report of the national arbovirus and malaria advisory committee. Commun Dis Intell Q Rep, 2016; 40(3):E400-E436 ×
Given that locally acquired cases of WNV in Australia have not been recorded, targeted testing only is recommended for potential donors with compatible symptoms (similar to flu) and a history of recent travel to an endemic country or an area with an ongoing outbreak. The incubation period for WNV is approximately 3–15 days, and infected individuals are viraemic for up to a week, therefore history of travel up to 4 weeks prior is of interest. If WNV is suspected, advice should be sought from an infectious disease specialist regarding testing requirements and how to proceed in the event of a positive test. Testing should be performed using NAT, using PCR at the current time.
Recommendation
Screening of asymptomatic donors for WNV is not recommended. Targeted testing using serology and NAT (PCR) is recommended for potential donors with compatible symptoms and a recent history of travel (<4 weeks prior) to an endemic country or a region with an ongoing outbreak. If a donor is suspected or known to be infected with WNV, an infectious disease specialist should be consulted for advice on testing requirements and whether it is safe to proceed with donation.
2.3.2.12 Zika virus
Australia and New Zealand do not have local transmission of Zika virus, but suitable mosquito vectors exist in some parts of northern Australia and near neighbours. Of confirmed/probable cases of Zika virus infection diagnosed in Australia and New Zealand in 2017, the majority of these cases were acquired in Tonga, Fiji, Samoa, Mexico or Brazil.125,126125 Summary information about overseas acquired vectorborne disease notifications in Australia. Australian Government Department of Health (http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-vectorborne-overseas-acquired.htm) 126 Zika virus infection weekly report: 27 February 2017. New Zealand Ministry of Health and the Institute of Environmental Science and Research Ltd. Available from https://surv.esr.cri.nz/surveillance/WeeklyZikaFever.php ×
Zika virus should be considered in potential donors with compatible symptoms and a history of recent travel (<4 weeks) to Zika-affected areas (https://wwwnc.cdc.gov/travel/page/zika-information) The median incubation period of Zika virus associated disease is 5.9 days, with 99% of infected individuals clearing the virus within 23.4 days.127 Although sexual contact with men who have been in an area of Zika virus transmission anytime in the prior 6 months is also theoretically a risk factor,128 sexual transmission of Zika virus is extremely uncommon in Australia or New Zealand. Screening for Zika virus in asymptomatic donors is not recommended. For donors with a history of recent travel to Zika virus-affected areas who do not have any symptoms of viral infection, the risk of Zika virus infection is very low and the consequences of Zika virus infection in transplant recipients have not been shown to be severe.129127 Lessler J, Ott CT, Carcelen AC, et al. Times to key events in Zika virus infection and implications for blood donation: a systematic review. Bull World Health Organ, 2016; 94(11):841-849. ×128 Zika virus – CDNA National Guidelines for Public Health Units. Australian Government Department of Health: https://www.health. gov.au/resources/publications/zika-virus-cdna-national-guidelines-for-public-health-units?language=en ×129 Nogueira ML et al. Zika virus infection and solid organ transplantation: a new challenge. Am J Transplant, 2017; 17(3): 791-795. ×
Recommendation
Screening of asymptomatic donors for Zika virus is not recommended. Zika serology should only be used as a diagnostic test in donors with compatible symptoms and epidemiological risk factors (i.e. travel to an endemic area within the previous 4 weeks). If the test is positive, seek advice from an infectious diseases specialist.
2.3.2.13 Other arboviruses
While the potential for donor-derived transmission exists, very little is known about the risks to organ transplant recipients of other arboviruses (dengue, chikungunya, Ross River virus, Murray Valley virus, Barmah forest virus, Japanese encephalitis). Importantly, arboviral infections are transient and there is no evidence for establishment of latency and latent disease. Donor testing is appropriate in the context of potential donors with compatible symptoms who have recently visited an endemic area. Targeted testing in this context would be warranted, typically with IgM, IgG and PCR, and the advice of an infectious disease specialist should be sought to guide appropriate testing and how to proceed in the event of a positive test. Decisions to proceed with transplantation should be made on a case by case basis, dependent on the organ(s) being considered for transplantation, availability of donor testing results prior to donation, and the nature of the infection.
Recommendation
If arboviral infection is suspected in a potential donor with compatible symptoms and a history of travel to an endemic area in the past 4 weeks, advice from an infectious disease specialist should be sought on appropriate testing procedures and what to do in the event of a positive test result.
2.3.3 Bacterial and fungal infections
2.3.3.1 Blood stream infections
Bacterial transmission through organ transplantation is probably common, as transient fever or infection with common organisms in recipients may not be recognised as donor-derived. An estimated 5% of organ donors have unrecognised bacteraemia at the time of donation, and abdominal contents are commonly contaminated during retrieval. Recipient outcomes are not adversely affected when the organisms are common, drug-sensitive pathogens and appropriate prophylactic antibiotics are administered. 130,131 When significant infection that is proven to be donor-derived does occur, it is more likely to be with resistant bacteria not covered by routine antibiotic prophylaxis or treatment in the donor and/or recipient (e.g. methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and multidrug resistant gram negative bacilli).9130 Kovacs Jr CS, Koval CE, van Duin D, et al. Selecting suitable solid organ transplant donors: Reducing the risk of donor-transmitted infections. World J Transplant 2014; 4(2): 43-56. 131 Fischer SA, Lu K, A.S.T. Infectious Diseases Community of Practice. Screening of donor and recipient in solid organ transplantation. Am J Transplant, 2013; 13(suppl 4):9-21 ×9 Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant, 2011;11:1123–1130. ×
The routine culturing of preservation fluids in which organs are transported is of uncertain benefit and is currently not recommended. Contamination of preservation fluids is common, however contaminants are rarely of clinical significance.132,133,134 In rare cases where contaminants are pathogenic, such organisms would typically be covered by routine prophylactic antibiotics.132 The burden of clinically irrelevant positive test results that would be generated by routine culturing of bag fluids would outweigh the benefits to recipients.132 Oriol I, Llado L, Vila M et al. The etiology, incidence, and impact of preservation fluid contamination during liver transplantation. PLOS One, 2016; 11(8): e0160701 133 Audet, M, Piardi T, Panaro F et al. Incidence and clinical significance of bacterial and fungal contamination of the preservation solution in liver transplantation. Transplant Infect Dis. 2010; 13: 84-88 134 Janny S, Bert F, Dondero F et al. Microbiological findings of culture-positive preservation fluid in liver transplantation.Transplant Infect Dis, 2010; 13: 9-14 ×132 Oriol I, Llado L, Vila M et al. The etiology, incidence, and impact of preservation fluid contamination during liver transplantation. PLOS One, 2016; 11(8): e0160701 ×
The culturing of preservation fluids would, however be indicated in exceptional circumstances. Digestive tract breach at the time of multiorgan recovery has been linked to multiple cases of graft-transmitted candidiasis in kidney and liver recipients, resulting in fungal arteritis in several cases.135 In the event of digestive tract rupture, appropriate specimens should be collected, including culture of organ preservation fluid, and surveillance cultures in the recipient where possible.135 Matignon M, Botterel F, Audard V et al. Outcome of renal transplantation in eight patients with Candida sp. Contamination of preservation fluid. Am J Transplant, 2008; 8:697 ×
Donors with endocarditis should be treated on a case-by-case basis in a manner similar to bacteraemic donors, with consideration given to the potential for septic emboli in transplantable organs.
There is little data regarding donors with fungaemia. Given the difficulty treating fungi, guiding principles would suggest they will generally be unsuitable, however if donation is being considered the case should be discussed with an infectious diseases specialist. The assessment would consider such factors as whether the infection is controlled, whether there are signs of dissemination to the organ, the options for treatment including those relevant to the specific organ (e.g. some antifungals do not penetrate the urinary tract). A full course of antifungal treatment of a minimum duration of two weeks should be given to the organ recipients, with culture surveillance for the development of active infection.
For recommendations in the case of bacterial meningitis, see Section 2.3.5 (Central Nervous System Infection).
Recommendation
Bacteraemia is not a contraindication to donation, and organs may be used after the donor has been treated with antibiotics. Recipients of organs from donors with confirmed bacteraemia should receive a full course of antibiotic treatment, with monitoring for evidence of infection. Donors with ongoing sepsis and persistently positive blood cultures should not be utilised. Organs from donors with endocarditis may be transplanted after the donor has been treated with antibiotics and after consideration of the risk of emboli to organs for transplantation. Cases of fungaemia should be discussed with an infectious diseases specialist before proceeding to donation.
2.3.3.2 Pulmonary infections
Bacterial colonisation of donor lungs is common as (1) the lungs are in constant contact with the external environment and the airways are normally colonised with multiple organisms, (2) most donors require emergency intubation, which may result in aspiration and pneumonia, and (3) the rate of bronchopulmonary infections increases in proportion to the length of time spent in the ICU. Prior to donation, aspiration and consequent pneumonia must therefore be ruled out or treated. In the case of pneumonia without bacteraemia, all other organs can be used safely. Following a period of antibiotic treatment and provided pulmonary function is not impaired, lungs may be considered for donation except where the pathogen is multi-drug resistant.
Invasive fungal infection of the lungs (including with endemic mycoses – e.g. histoplasmosis, coccidiodomycosis, blastomycosis) is very uncommon and the advice of an infectious diseases specialist should be sought in this situation if transplantation is being considered. Much more common is fungal colonisation of the donor airway, which is managed by routine antifungal prophylaxis and/or pre-emptive antifungal therapy according to unit policy.
Recommendation
In the case of pneumonia without bacteraemia, all other organs can be used safely for transplant. Lungs may be used after adequate and effective antibiotic therapy, with a full antibiotic course administered to the recipient. Fungal colonisation of the donor airway is not a contraindication to donation; however, in the case of invasive fungal infection the advice of an infectious diseases specialist should be sought.
2.3.3.3 Urinary tract infections
Urinary tract infection (UTI), with the risk of pyelonephritis, is common among potential donors due to bacteria or fungi ascending along the urethral catheter. Any suspected UTIs in potential donors should be confirmed by urine culture.
Prior to organ retrieval, the donor should be treated with antibiotics.121 The final decision about organ utilisation should be made at the time of organ retrieval. Post-transplant treatment of the recipient is expected to reduce the risk of donor-derived infection. In general, however, there is no need to treat the recipient of a non-kidney organ from a deceased donor with nonbacteraemic, localised infection that does not involve the transplanted organ.121 https://www.who.int/news-room/fact-sheets/detail/west-nile-virus ×
Candida infection early post kidney transplant has been associated with death and morbidity such as vascular and anastamotic complications.136 Candida in the urinary tract of the donor is commonly thought to arrive there from contamination by faeces from intestinal perforation, directly infecting the kidneys from the external capsule then progressing inside the kidney. Culture of preservation fluids in cases where a breach of the digestive tract is identified during organ recovery is recommended for the early detection of Candida sp. Where donor or early recipient urinary tract cultures are positive for Candida sp. ongoing surveillance for complications should occur and antifungal therapy given to the recipient for a minimum 2 weeks.136 Albano L, Bretagne S, Mamzer-Bruneel MF et al. Evidence that graft-site candidiasis after kidney transplantation is acquired during organ recovery: a multicentre study in France. Clin Infect Dis, 2009; 48(194) ×
Recommendation
In the case of UTI without bacteraemia, all non-kidney organs can be used safely for transplant. Uncomplicated UTI/bacteruria is in most cases not a contraindication to utilisation of kidneys if there has been adequate and effective antibiotic treatment (within time constraints of donation suitability assessment) and a full antibiotic treatment course is administered to the recipient. Where donor or early recipient urinary tract cultures are positive for Candida, ongoing surveillance for complications should occur and antifungal therapy be given to the recipient for a minimum of 2 weeks.
2.3.3.4 Multi-drug resistant bacteria
Donor exposure to multi-drug resistant (MDR) bacteria in the ICU is an increasing problem, in particular exposure to vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, extended-spectrum beta- lactamase (ESBL)-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, multidrug-resistant Pseudomonas aeruginosa, and carbapenem-resistant Acinetobacter baumanni. Prolonged ICU stay, vasopressor use, need for cardiopulmonary resuscitation and injuries involving major blood loss increase the risk of infection or colonisation with MDR bacteria (although nosocomial infections may be acquired even with only a short hospital stay).137,138 In addition, significant volume and blood product replacement in the donor with traumatic injury may result in a wash-out effect of prophylactic antibiotics and ineffective antibiotic coverage.138 Donor country137 Wu TJ, Lee CF, Chou HS, et al. Suspect the donor with potential infection in the adult deceased donor liver transplantation. Transplant Proc, 2008; 40(8):2486-8. 138 Watkins AC, Vedula GV, Horan J, et al. The deceased organ donor with an “open abdomen”: proceed with caution. Transpl Infect Dis, 2012; 14(3):311-5 ×138 Watkins AC, Vedula GV, Horan J, et al. The deceased organ donor with an “open abdomen”: proceed with caution. Transpl Infect Dis, 2012; 14(3):311-5 ×
of origin/prior residence is also a potential risk factor: donors from countries with high rates of gut colonisation with multi-drug-resistant bacteria pose a higher risk of transmission, as do donors who have previously been hospitalised overseas. Detection of MDR bacteria may be difficult due to antibiotic therapy, which may reduce the bacterial load to a level that is undetectable by standard culture protocols but is still able to transmit infection to an immunosuppressed individual.139139 Orlando G, Di Cocco P, Gravante G, et al. Fatal haemorrhage in two renal graft recipients with multi-drug resistant Pseudomonas aeruginosa infection. Transpl Infect Dis, 2009; 11(5):442-7. ×
At this time there is no need for enhanced microbiological screening of potential donors for MDR bacteria over and above standard ICU practice. If MDR bacteria are identified prior to transplantation, the risks are highest for the bacteraemic donor or where the positive culture is taken from the organ that is to be transplanted: in these cases transplantation should be carefully considered and advice sought from an infectious diseases physician.140 In all other circumstances, transplantation can be considered in consultation with an infectious diseases physician.140 Colonisation by MDR bacteria is not a contraindication for donation provided the colonised tissue remains sealed from the rest of the body and any adjacent organs are not affected.140 Mularoni A, Bertani A, Vizzini G et al. Outcome of transplantation using organs from donors infected or colonized with carbapenem-resistant gram-negative bacteria. Am J Transplant, 2015; 15: 2674-2682. ×140 Mularoni A, Bertani A, Vizzini G et al. Outcome of transplantation using organs from donors infected or colonized with carbapenem-resistant gram-negative bacteria. Am J Transplant, 2015; 15: 2674-2682. ×
Recommendation
Organs from donors with positive cultures for MDR bacteria may be considered for transplantation with close recipient follow-up. Transplantation should be carefully considered in the event that the organ to be transplanted is itself colonised or the donor is bacteremic. A key consideration is whether safe antibiotic options are available to treat the bacterium. The case should be discussed with an infectious diseases physician and if donation proceeds, a full treatment course should be administered to the recipient.
2.3.3.5 Mycobacterium tuberculosis
The vast majority of tuberculosis cases in Australia and New Zealand occur in the overseas-born population, with other major risk factors including household or close contact with tuberculosis, employment in the health industry, incarceration, residence in an aged care facility, homelessness, and immunosuppression.141 In the general Australian population, pulmonary tuberculosis accounts for approximately 60% of tuberculosis cases, with 40% being extrapulmonary.141141 Toms C, Stapledon R, Waring J, et al. Tuberculosis notifications in Australia, 2012 and 2013. Commun Dis Intell Q Rep, 2014: 39(2):E217-35. ×141 Toms C, Stapledon R, Waring J, et al. Tuberculosis notifications in Australia, 2012 and 2013. Commun Dis Intell Q Rep, 2014: 39(2):E217-35. ×
Reasonable efforts should be made to rule out active tuberculosis in donors with any epidemiological risk factors for tuberculosis or history of tuberculosis infection. However, there are no proven methods for screening deceased donors for tuberculosis. Chest X-ray and direct microscopy of bronchoalveolar lavage for acid-fast bacilli have a low sensitivity, and cultures may take up to eight weeks to turn positive.142 Tuberculin skin testing and interferon gamma release assays are also impractical in the context of deceased donation given their slow turn-around times. NAT can identify M. tuberculosis in clinical specimens from donors with active infection only; a negative result does not definitively rule out infection with M. tuberculosis, as organisms can remain dormant in the host without causing disease for decades, without any detectable radiographic abnormality. Conversely, abnormal pulmonary findings from a range of causes are common in deceased donors and may confound donor evaluation.142142 Morris MI, Daly JS, Blumberg E et al. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am J Transplant, 2012; 12(9):2288-2300. ×142 Morris MI, Daly JS, Blumberg E et al. Diagnosis and management of tuberculosis in transplant donors: a donor-derived infections consensus conference report. Am J Transplant, 2012; 12(9):2288-2300. ×
Given the limitations of tuberculosis screening tools in deceased donors, and given that it is unclear how to proceed on the basis of results from such screening, routine donor screening for tuberculosis is not recommended. Donor screening for tuberculosis can be triggered by the TB screening questions within the electronic donor record AUS-DRAI. Diagnostic testing for tuberculosis is recommended where there is clinical suspicion of tuberculosis infection that is supported by epidemiological factors.
Recommendation
Diagnostic testing for tuberculosis with microscopy (acid-fast bacillus staining) and PCR are recommended where infection is suspected based on epidemiological AND clinical factors that are suggestive of either active or latent tuberculosis. Donation of organs from donors currently being treated for tuberculosis or with positive results (e.g. AFB stain, NAT) is not recommended other than in exceptional circumstances after discussion with an infectious diseases physician. Donors with previous active or latent tuberculosis can be considered, taking into account tuberculosis antibiotic susceptibilities, completeness of donor treatment, and current evidence of infection in the organ. Discussion with an infectious diseases physician, close follow up of the recipient and consideration of tuberculosis prophylaxis for the recipient are recommended.
2.3.3.6 Treponema pallidum (syphilis)
is negative, then a second treponemal test based on different antigens to the original test should be performed. This approach differentiates potential donors who have been previously treated for syphilis from those with untreated
The stage of syphilis needs to be considered in donors with positive syphilis serology and the case should be discussed with an infectious diseases physician. If secondary syphilis is likely, then the infection may be disseminated and donation should probably not proceed apart from exceptional circumstances. The possibility of tertiary syphilis of the aortic arch should be considered in the case of heart donation. For donors deemed to have primary, latent, or tertiary syphilis, donation may proceed with benzathine or intravenous penicillin prophylaxis given to the recipients with a regimen advised by an infectious diseases physician.
The presence of newly diagnosed syphilis in the donor indicates the donor is at increased risk of having also recently acquired HIV, HBV or HCV, and decisions regarding utilisation should be made accordingly.
Recommendation
If primary, latent or tertiary syphilis is detected in the donor, donation may proceed with appropriate prophylactic treatment of the recipient. A donor with secondary syphilis may be bacteraemic with the involvement of many organs, hence caution should be taken if clinical manifestations of secondary syphilis are present.
2.3.4 Parasitic infections
2.3.4.1 Malaria (Plasmodium sp)
Australia and New Zealand remain free of endemic malaria: all notifications are in travellers or those who haveresided in endemic regions. Despite the absence of endemic malaria, suitable vector mosquitos for Plasmodium sp are present in northern Australia and the area is "malaria receptive". Limited transmission does also sometimes occur in the Torres Strait following importation.149149 Bright, Amy, NNDSS Annual Report Working Group, Matthew R O’Dwyer, and Mark Trungove. 2021. “Australia’s Notifiable Disease Status, 2016: Annual Report of the National Notifiable Diseases Surveillance System”. Communicable Diseases Intelligence 45 (May). https://doi.org/10.33321/cdi.2021.45.28. ×
There are several Plasmodium spp. relevant to humans including Plasmodium falciparum, Plasmodium ovale, Plasmodium vivax, Plasmodium malariae and Plasmodium knowlesi.150 Certain species of Plasmodium with partial immunity can allow development of asymptomatic parasitaemia. P.ovale and P. vivax have a hypnozoite phase in the liver that can reactivate into a blood stage years after initial infection. While P.malariae does not have a hypnozoite stage, it can cause illness years after leaving an endemic region.151 - 153150 Daily JP, Minuti A, Khan N. Diagnosis, treatment, and prevention of malaria in the US: a review. Jama. 2022 Aug 2;328(5):460-71. ×151 Official Journal of the European Union. Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/ EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/ WC500004484.pdf. Published March 30, 2004 152 Therapeutic Goods Administration. Therapeutic goods (standards for human cell and tissue products-donor screening requirements) (TGO108) order 2021. Canberra: Department of Health; 2021. Therapeutic Goods (Standard for Human Cell and Tissue Products—Donor Screening Requirements) (TGO 108) Order 2021 - Federal Register of Legislation 153 FDA Guidance Document, Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria. January 2025. https://www. fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-reduce-risk-transfusion-transmitted-malaria-0 ×
Although malaria is a rarely reported complication of organ transplantation outside of non-endemic countries, internationally and in Australia there have been a small number of documented cases of donor-derived malaria transmission involving all solid organ transplant recipients, frequently from donors with unrecognised asymptomatic parasitaemia involving Plasmodium spp. with a hypnozoite phase.154 - 164 When donor-derived transmission does occur, if detected early it can be treated effectively.165,166,167,168154 Pierrotti LC, Levi ME, Di Santi SM, Segurado AC, Petersen E. Malaria Disease Recommendations for Solid Organ Transplant Recipients and Donors. Transplantation. 2018 Feb;102(2S Suppl 2):S16-S26. doi: 10.1097/TP.0000000000002017. PMID: 29381574. 155 Martín-Dávila P, Norman F, Fortún-Abete J, Píris M, Lovatti R, Rubio JM, Martinez-Pérez A, Graus J, Ta G, Villarubia J, Mahillo B, López-Vélez R. Donor-derived multiorgan transmission of mixed P. malariae and P. ovale infection: Impact of globalization on post-transplant infections. Transpl Infect Dis. 2018 Oct;20(5):e12938. 156 Boissier E, Lakhal K, Lepoivre T. Donor-imported and transmitted malaria after lung transplantation. Br J Haematol. 2020 Aug;190(4):485. 157 Arispe Angulo KR, Harrington AM. Fever in a kidney transplant patient From Nigeria. The Hematologist. 2020 Jan 1;17(1). 158 Vernaza A, Pinilla‐Monsalve G, Cañas F, Carrillo D, David Lopez J, Flórez N, Gomez‐Mesa JE. Malaria and encephalopathy in a heart transplant recipient: A case report in the context of multiorgan donation. Transplant Infectious Disease. 2021 Jun;23(3):e13565. 159 Rosso F, Agudelo Rojas OL, Suarez Gil CC, Lopez Vargas JA, Gómez‐Mesa JE, Carrillo Gomez DC, Meza Ramirez L, Caicedo Rusca LA. Transmission of malaria from donors to solid organ transplant recipients: a case report and literature review. Transplant Infectious Disease. 2021 Aug;23(4):e13660. 160 Shendi AM, Aly AA, Alsaad KO, Shah Y, Ali TZ, Broering DC, Aleid HA. Thrombotic microangiopathy after kidney transplantation: lessons from a transplant tourist. Journal of Nephrology. 2023 May;36(4):1191-5. 161 O’Leary M, Ana D, Lee R, Cavazzoni E, Finemore T, Watts MR, Stelzer-Braid S, Rawlinson W. 242.4: Donor-derived transmission of malaria–Implications for donor screening. Transplantation. 2024 Sep 1;108(9S). 162 Ushiro-Lumb I, Geoghegan C, Smit M, Kothalawala R, Maddox V, Webster M. P. 133: Identification of risk of malaria and selective post-transplant testing of deceased organ donors in the UK. Transplantation. 2024 Sep 1;108(9S). 163 Rodríguez-Gutiérrez AF, Ramírez-Sánchez IC. Malaria after liver transplantation: Report of two cases and a review of published cases. Biomedica: revista del Instituto Nacional de Salud. 2025 May 30;45(2):180-9. 164 Krendl FJ, Resch T, Eschertzhuber S, Schneeberger S, Oberhuber R. Normothermic liver machine perfusion as a dynamic platform for assessment and treatment of organs from a donor with malaria–expanding the indications. Journal of Hepatology. 2024 Nov 1;81(5):e236-7. ×165 La Hoz RM, Morris MI; on behalf of the Infectious Diseases Community of Practice of the American Society of Transplantation. Tissue and blood protozoa including toxoplasmosis, Chagas disease, leishmaniasis, Babesia, Acanthamoeba, Balamuthia, and Naegleria in solid organ transplant recipients— Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019; 33:e13546. https://doi.org/10.1111/ctr.13546 166 Ushiro-Lumb I, Geoghegan C, Smit M, Kothalawala R, Maddox V, Webster M. P. 133: Identification of risk of malaria and selective post-transplant testing of deceased organ donors in the UK. Transplantation. 2024 Sep 1;108(9S). 167 Len O, Los-Arcos I, Aguado JM, Blanes M, Bodro M, Carratala J, Cordero E, Fariñas MC, Fernandez-Ruiz M, Fortun J, Gavalda J. Selection criteria of solid organ donors in relation to infectious diseases: a Spanish consensus. Transplantation Reviews. 2020 Apr 1;34(2):100528. 168 Bansal SB, Ramasubramanian V, Prasad N, Saraf N, Soman R, Makharia G, Varughese S, Sahay M, Deswal V, Jeloka T, Gang S. South Asian Transplant Infectious Disease Guidelines for solid organ transplant candidates, recipients, and donors. Transplantation. 2023 Sep 1;107(9):1910-34. ×
Thick and thin blood smears and rapid diagnostic antigen tests (RDTs) are insufficiently sensitive to exclude asymptomatic parasitaemia in donors. Nucleic acid tests (NATs) have high sensitivity for asymptomatic parasitaemia in most donors. Antibody testing is highly sensitive but does not differentiate prior from current infection. No diagnostic test is perfectly sensitive so in the event recipients develop a compatible clinical syndrome theyshould be investigated for malaria.162,169,170162 Ushiro-Lumb I, Geoghegan C, Smit M, Kothalawala R, Maddox V, Webster M. P. 133: Identification of risk of malaria and selective post-transplant testing of deceased organ donors in the UK. Transplantation. 2024 Sep 1;108(9S). 169 O’Leary M, Ana D, Lee R, Cavazzoni E, Finemore T, Watts MR, Stelzer-Braid S, Rawlinson W. 242.4: Donor-derived transmission of malaria–Implications for donor screening. Transplantation. 2024 Sep 1;108(9S). 170 Seed CR, Coughlin JT, Pickworth AM, Harley RJ, Keller AJ. Relapsing vivax malaria despite chemoprophylaxis in two blood donors who had travelled to Papua New Guinea. Medical journal of Australia. 2010 Apr 19;192(8). ×
RecommendationAll donors who have spent more than 3 months in a malaria endemic region, or who have visited (any duration) amalaria endemic region in the last 12 months (https://www.who.int/data/gho/data/themes/malaria), should be screened for Plasmodium infection using NAT or serology.Retrospective results are satisfactory. If NAT or serology is positive, transplantation may proceed, and recipientsshould be treated (and tested) for malaria. If donor testing is negative, recipients should be tested for malariashould compatible clinical syndrome develop.
2.3.4.2 Strongyloides stercoralis
Strongyloides is an intestinal nematode that is endemic to tropical or subtropical regions of the world. Once infection occurs, the Strongyloides parasite establishes an autoinoculation cycle that allow infection to persist in the host indefinitely. Infection is transmitted by skin contact with soil contaminated with human waste, and prevalence is therefore directly related to sanitation and hygiene conditions. Outside of the endemic regions of Southeast Asia, Central and South America, and Africa, Strongyloides infection is also found in poor communities, former war veterans, refugees, immigrants and travellers, and people occupationally exposed to soil (e.g. farmers and miners) in parts of the United States, Europe, United Kingdom, and Australia.171,172 Groups with high rates of Strongyloides infection in Australia include war veterans, immigrants/refugees, and Aboriginal and Torres Strait Islander Australians (particularly children).173,174,175,176,177 Infection with HTLV-1 is associated with increased prevalence of S. stercoralis infection.178,179171 Beknazarova M, Whiley H and Ross K. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health, 2016; 13(5). 172 Annette Olsen, Lisette van Lieshout, Hanspeter Marti, et al. Strongyloidiasis – the most neglected of the neglected tropical diseases?, Transactions of The Royal Society of Tropical Medicine and Hygiene, Volume 103, Issue 10, October 2009, Pages 967–972. ×173 Rahmanian H, MacFarlane AC, Rowland KE et al. Seroprevalence of Strongyloides stercoralis in a South Australian Vietnam veteran cohort. Aust NZ J Public Health, 2015; 39(4): 331-5. 174 Shield J, Braat S, Watts M et al. (2021) Seropositivity and geographical distribution of Strongyloides stercoralis in Australia: A study of pathology laboratory data from 2012–2016. PLoS Negl Trop Dis 15(3):e0009160. 175 Caruana SR, Kelly HA, Ngeow JY et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med, 2006; 13(4):233-9. 176 de Silva S, Saykao P, Kelly H et al. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7-20 years after resettlement in Australia. Epidemiol Infect, 2002; 128(3);439-444. 177 Chaves NJ, Paxton GA, Biggs BA et al. The Australasian Society for Infectious Diseases and Refugee Health Network of Australia recommendations for health assessment for people from refugee-like backgrounds: an abridged outline. Med J Aust. 2017 Apr 17;206(7):310-315. ×178 Talukder MR, Pham H, Woodman R et al. The Association between Diabetes and Human T-Cell Leukaemia Virus Type-1 (HTLV-1) with Strongyloides stercoralis: Results of a Community-Based, Cross-Sectional Survey in Central Australia. Int J Environ Res Public Health. 2022 Feb 13;19(4):2084. 179 Gordon CA, Shield JM, Bradbury RS, et al. HTLV-I and Strongyloides in Australia: The worm lurking beneath. Adv Parasitol. 2021;111:119-201. ×
Donor screening with serology, using Strongyloides IgG EIA, is recommended for all donors due to the difficulty obtaining an accurate history for risk of exposure. Infection requires a period of residence in an endemic area (e.g. three months or more) and because of the longevity of the parasitic infection, screening is warranted even for very remote histories of travel to endemic regions.180 Strongyloides testing may be retrospective, given that there is a window post-transplant in which infection can be treated effectively, although results should still be provided as soon as possible.180 White SL, Rawlinson W, Boan P, Sheppeard V et al. Infectious Disease Transmission in Solid Organ Transplantation: Donor Evaluation, Recipient Risk, and Outcomes of Transmission. Transplant Direct. 2018 Dec 20;5(1):e416 ×
Recommendation
All potential donors should be tested for Strongyloides. Retrospective results are satisfactory and can guide recipient management post-operatively. If the donor tests positive, recipients should receive prophylactic treatment with ivermectin.181181 Henriquez-Camacho C, Gotuzzo E, Echevarria J et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016 Jan 18;2016(1). ×
2.3.4.3 Toxoplasma gondii
Exposure to Toxoplasma gondii is extremely common in all parts of the world, including Australia. A study of pregnant women in Australia found 35% had IgG antibodies to T. gondii.182 Following infection, T. gondii spreads to organs and tissues and is able to multiply in almost any cell in the body.183 Immunity does not eradicate the infection, as latent intracellular cysts can persist for years after acute infection mainly in muscle tissues and the brain (although visceral organs may also be infected).183 Both acute and latent T. gondii infection in the donor pose a transmission risk, and T. gondii transmission by organ transplantation has been reported multiple times in the literature, most commonly by heart transplantation but also by kidney liver, bowel and pancreas transplantation.184,185,186,187,188,189,190182 Walpole IR, Hodgen N and Bower C. Congenital toxoplasmosis: a large survey in Western Australia. Med J Aust, 1991; 154(11): 720-724. ×183 Hill DE, Chirukandoth S and Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev, 2005; 6(1): 41-61. ×183 Hill DE, Chirukandoth S and Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev, 2005; 6(1): 41-61. ×184 Rogers NM, Peh CA, Faull R et al. Transmission of toxoplasmosis in two renal allograft recipients receiving an organ from the same donor. Transpl Infect Dis, 2008; 10(1):71-74. 185 Giordano LF and Lasmar EP. Toxoplasmosis transmitted via kidney allograft: case report and review. Transplant Proc, 2002; 34(2): 498-499. 186 Renoult E, Georges E, Biava MF et al. Toxoplasmosis in kidney transplant recipients: report of six cases and review. Clin Infect Dis, 1997; 24(4): 625-634. 187 Mason JC, Ordelheide KS, Grames GM et al. Toxoplasmosis in two renal transplant recipients from a single donor. Transplantation, 1987; 44(4): 588-591. 188 Fernandez-Sabe N, Cervera C, Farinas MC et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis, 2012; 54(3): 355-361. 189 Campbell AL, Goldberg CL, Magid MS et al. First case of toxoplasmosis following small bowel transplantation and systematic review of tissue-invasive toxoplasmosis following non-cardiac solid organ transplantation. Transplantation, 2006; 81(3): 408-417. 190 Hommann M, Schotte U, Voigt R et al. Cerebral toxoplasmosis after combined liver pancreas-kidney and liver pancreas transplantation. Transplant Proc, 2002; 34(6): 2294-2295. ×
Numerous serological tests exist for the detection of T. gondii antibodies, including both IgM and IgG. IgM antibodies appear sooner after infection than IgG, and disappear following recovery (whereas IgG antibodies do not disappear). NAT can be used to diagnose active infection;191,192 however, given that active infection is rare and the goal of donor screening is primarily to detect latent toxoplasma in the heart and other organs resulting from past infection, serological testing for toxoplasma IgG alone is recommended, with testing for acute toxoplasma (IgM, NAT) reserved for appropriate clinical scenarios.191 Lewis JS Jr, Khoury H, Storch GA and DiPersio J. PCR for the diagnosis of toxoplasmosis after hematopoietic stem cell transplantation. Expert Rev Mol Diagn, 2002; 2(6): 616-624. 192 Joseph P, Calderón MM, Gilman RH et al. Optimization and evaluation of a PCR assay for detecting toxoplasmic encephalitis in patients with AIDS. J Clin Microbiol, 2002; 40(12): 4499-4503. ×
While a positive serological test for T. gondii is not a contraindication to donation, it may inform the need for prophylaxis in the recipient. Trimethoprim-sulfamethoxazole prophylaxis for at least 3 months post-transplant is currently standard international practice for recipients at risk of T. gondii transmission.193193 Coster LO. Parasitic infections in solid organ transplant recipients. Infect Dis Clin North Am, 2013; 27(2): 395-427. ×
Recommendation
Toxoplasma gondii screening using serology is recommended for all potential donors, with results available either prospectively or retrospectively. For recipients at risk (donor and/or recipient seropositive), routine Pneumocystis jiroveci prophylaxis with cotrimoxazole is protective against toxoplasmosis. If cotrimoxazole is not tolerated, prophylaxis should be chosen which is active against Pneumocystis and Toxoplasma gondii (e.g. atovaquone, or dapsone plus pyrimethamine, not pentamidine).
2.3.4.4 Trypanosoma cruzi
Trypanosoma cruzi (Chagas disease) is a zoonotic protozoan endemic to Mexico, Central America and South America.194 Following infection, trypomastigotes circulate in the blood stream, while intracellular amastigotes appear in muscle (including heart) and ganglion cells.195 Acute infection is typically asymptomatic, with an initial period of high parasitaemia followed by chronic latent infection, which then progresses to cardiac, gastrointestinal and/or peripheral nervous system disease in approximately 30% of those infected.195 In immunosuppressed persons, acute T. cruzi infection is associated with adverse outcomes, particularly where there is cardiac and/or central nervous system involvement.195194 Chin-Hong PV et al. Screening and treatment of chagas disease in organ transplant recipients in the United States: recommendations from the chagas in transplant working group. Am J Transplant, 2011; 11(4): 672-680. ×195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×
Routine travel to endemic regions carries a low risk of T. cruzi infection; at risk are those who have spent prolonged time in endemic areas (>3 months) and/or stayed in rural/disadvantaged areas. The risk of transmission from T. cruzi seropositive organ donors to seronegative recipients ranges from 10-20% for kidneys and livers, to >75% for hearts.195 Evidence indicates kidneys and livers can be safely transplanted from T. cruzi positive donors if close post-transplant monitoring is in place for early diagnosis and treatment, should acute infection occur.195 Recipients of T. cruzi positive organs should be monitored weekly for the first 2 months post transplant, every 2 weeks through months 3-6 and annually thereafter or after intensification of immunosuppression. Monitoring methods include blood microscopy for T. cruzi, blood nucleic acid tests, and serology. Given the high rate of transmission in the context of heart transplantation, hearts from donors infected with or screen-positive for T.cruzi should not be utilised.194195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×194 Chin-Hong PV et al. Screening and treatment of chagas disease in organ transplant recipients in the United States: recommendations from the chagas in transplant working group. Am J Transplant, 2011; 11(4): 672-680. ×
Chronic T. cruzi infection should be diagnosed on the basis of epidemiological factors and serological tests, using at least 2 serological methods (e.g. ELISA, indirect haemagglutination assay, indirect immunofluorescence assay) and with inconclusive results confirmed by PCR. Serological tests for T.cruzi have good sensitivity but poor specificity in chronically infected persons, whereas PCR has high specificity and low sensitivity.195 Serological results are unlikely to be available within the donation timeframe but can inform post-transplant interventions.11 Monitoring for evidence of transmission and prompt treatment of acute infection is preferred to the use of prophylaxis in D+/R- transplants.195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×11 Ison, M.G., P. Grossi, and A.S.T Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant, 2013. 13 Suppl 4: p. 22-30. ×
Recommendation
Donors who have spent 3 months or more in Mexico, Central or South America at any time in their lives should be screened for T.cruzi using serology. Infection with T. cruzi is not a contraindication to the donation of non-cardiac organs, however recipients require close follow-up for 24 months post-transplant for the appearance of acute infection. Donors with known T.cruzi infection (acute or chronic infection) should be excluded from heart donation.
2.3.5 Central nervous system infection by various pathogens
Most central nervous system (CNS) infection is bacterial or viral meningitis and/or encephalitis. Individuals with meningitis and/or encephalitis sometimes deteriorate to the point of neurological death as a result of these infections, at which point they might be considered for organ donation.
Cases of donor-transmitted CNS infection reported in the international literature have also involved more unusual infectious agents, including Mycobacterium tuberculosis, lymphocytic choriomeningitis virus, rabies, cryptococcus, coccidioides immitis, aspergillus, and balamuthia, resulting in significant morbidity and mortality in recipients. 196 In some cases of donor-derived transmission, CNS infection was not suspected due other pathology such as trauma, stroke and hypoxic-ischaemic brain injury. Suspicion of any of the above agents as the cause of CNS infection in a potential donor should preclude donation.196 Kaul D, Covington S, Taranto S, et al. Solid organ transplant donors with central nervous system infection. Transplantation, 2014; 98(6):666-70. ×
By comparison, donors with microbiologically proven bacterial meningitis (e.g. Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Escherichia coli, or group B streptococcus) are acceptable if the donor has been receiving appropriate antibiotic therapy (ideally for 48 hours) and pathogen-directed prophylaxis is provided to the recipient. Donors infected with highly virulent organisms such as Listeria species should be excluded.197197 Skogberg K, Syrjanen J, Jahkola M et al. Clinical presentation and outcomes of listeriosis in patients with and without immunosuppressive therapy. Clin Infect Dis, 1992; 14(4): 815-821. ×
Patients with viral encephalitis should generally be excluded as donors, given the potentially fatal risk of transmission of pathogens such as Murray Valley encephalitis, lymphocytic choriomeningitis virus, and bat-related lyssavirus.195 In the case of encephalitis caused by HSV or Varicella-zoster virus (VZV), provided that the donor has received a period of antiviral treatment and is not viraemic, transplantation may proceed on a case-by-case basis. Recipients in this circumstance should receive a course of antivirals.195 Pierrotti LC, Carvalho NB, Amorin JP et al. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation, 2018; 102(2S Suppl 2): S1-S7. ×
Given that in some reports of donor-derived disease transmission CNS infection was not suspected, the following should raise the suspicion of possible CNS infection:196196 Kaul D, Covington S, Taranto S, et al. Solid organ transplant donors with central nervous system infection. Transplantation, 2014; 98(6):666-70. ×
Stroke in a patient without risk factors (e.g. young or paediatric, without comorbidities such as diabetes, hypertension or prior cerebrovascular accident) or clear mechanism
Fever early in presentation, or other features of CNS infection such as altered mental status or seizures (note that fever after hospitalisation in common in critically ill patients)
Travel or contact history posing a risk of CNS infection (e.g. travel to endemic regions for WNV or recent bat contact)
Donor is immunosuppressed either through medication or disease (e.g. autoimmune disease, cirrhosis or previous transplant)
Cerebrospinal fluid pleocytosis with decreased glucose and increased protein.
If suspicious of the presence or uncertain of the cause of CNS infection, a lumbar puncture should be performed followed by polymerase chain reaction (PCR) and other rapid diagnostic techniques.
Recommendation
Donors should be carefully evaluated for the possibility of CNS infection, with the cause established wherever possible. Donors with microbiologically proven bacterial meningitis are generally suitable for transplantation, provided the donors has been treated with appropriately targeted antibiotic therapy and the recipients receive targeted prophylaxis. Potential donors with viral encephalitis should not be utilised, with the exception of proven and treated HSV/VZV encephalitis. In the latter case, transplantation may proceed on a case-by-case after a period of antiviral treatment in the donor and with recipients receiving a course of antivirals post-transplantation. Donors with CNS infection of unknown origin must not be utilised.
2.3.5.1 Transmissible Spongiform Encephalopathies
Transmissible Spongiform Encephalopathies (TSEs) are a group of rare, transmissible, and lethal neurodegenerative disorders that can occur sporadically, due to genetic causes, or due to exposure to the transmissible agent (prion). Creutzfeld-Jakob disease (CJD) is the most common human TSE, and can occur in both sporadic (sCJD) and acquired (vCJD) forms. In the hospital setting, sCJD has been transmitted through medical or surgical procedures involving neurosurgical instruments, brain electrodes, tissue (human cornea and dura mater grafts) and tissue extracts (human pituitary hormones).198 While there have been no known transmissions of vCJD via surgery or tissue or organ donation to date, there have been cases of vCJD transmission via transfusion of red blood cells and plasma.199198 Guidance on the microbiological safety of human organs, tissues and cells used in transplantation. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2011. ×199 Guidance on the microbiological safety of human organs, tissues and cells used in transplantation. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2011. ×
Prospective CJD surveillance in Australia has been performed since 1993. Persons with suspected CJD are notified to the Australian National Creutzfeldt-Jakob Disease Registry, typically as a result of referral for diagnostic cerebrospinal fluid 14-3-3 protein detection, or alternatively via personal communications from clinicians, hospitals, families or CJD-related groups, and through health record searches.200 The CJD mortality rate in Australia is <2 per million population per year.200 Acquired CJD has not been reported in Australia to date.200 Klug GM, Boyd A, Sarros S et al. Creutzfeldt-Jakob disease surveillance in Australia: update to December 2015. Commun Dis Intell, 2016; 40(3): E368-E376. ×200 Klug GM, Boyd A, Sarros S et al. Creutzfeldt-Jakob disease surveillance in Australia: update to December 2015. Commun Dis Intell, 2016; 40(3): E368-E376. ×
There is currently no minimally invasive test to detect TSE before the onset of symptoms, nor is the prevalence of asymptomatic TSE known. Definitive diagnosis can only be made, if at all, by neuropathological examination of brain tissue following biopsy or autopsy. In the context of deceased organ donation, minimising the risk of donor- derived TSE transmission relies on screening the patient’s history for symptoms consistent with TSE, exposure to human blood, dura mater grafts, pituitary-derived hormones, contact with contaminated surgical instruments and/or prior notification from the department of health as being at increased-risk of TSE due to exposure to one or more risk factors.
The following people are at risk of TSE and should be excluded from the donation of organs and tissues (including blood and plasma):201201 Infection Control Guidelines: Creutzfeldt-Jakob disease. Australian Government Department of Health (http://www.health.gov.au/ internet/main/publishing.nsf/Content/icg-guidelines-index.htm) ×
People with a family history of CJD
People who received human pituitary-derived hormones prior to 1986
People who have received dura mater grafts, contact with contaminated surgical instruments, and/or prior notification from the department of health as being at increased risk of TSE due to exposure to one or more risk factors
People who die with early onset dementia
People who die with any obscure undiagnosed neurological disorder.
Residence in the in the United Kingdom for six months or more between 1980 to 1996 is NOT an exclusion for deceased organ donation in Australia or New Zealand.
Recommendation
Persons at risk of TSE (as defined above) should be excluded from organ donation.
2.4 Risk of donor transmitted malignancy
Active malignancy generally precludes organ donation, with some exceptions. Transmission of malignancy may nonetheless occur as a result of occult malignancy in the donor, or as a result of past or current malignancy that was judged to have a low chance of transmission at the time of donor evaluation. In these circumstances, transmission of cancer from donor to recipient is believed to occur in <0.1% of solid organ transplants.202202 Transplantation of Organs from Deceased Donors with Cancer or a History of Cancer. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2014. ×
Some data on the occurrence of malignancy transmission through organ transplantation are available through tumour registries and other databases (see Appendix J). These data have significant limitations due to incomplete capture of adverse events and the inherent difficulties of attributing cancer in the recipient to donor origin. Registries such as the Israel Penn International Transplant Tumor Registry (IPITTR) rely on voluntary event-based reporting and lack routine follow-up data on all recipients therefore tend to over-estimate risk. Conversely, transplant recipient databases may incompletely track recipients over time and thus under-report the development of cancer, resulting in under-estimation of risk. There are currently no transplant registries with systems in place to capture all cancer diagnoses over the lifetime of the recipient. Nor is there a system to capture instances where donor cancer has not resulted in transmission to the recipient, further complicating the calculation of transmission risk.
When a recipient develops a cancer – even when the cancer is proven to be of donor origin (by HLA or other molecular typing technology) – it is not necessarily the case that the cancer was donor-transmitted, since the transplanted organ may have developed the cancer subsequent to transplantation. That is, cancer in the recipient may be donor-derived but not donor-transmitted. Known examples include late renal cell carcinoma many years post-transplant and cases of donor-origin lymphoma that are likely to have developed through EBV transformation of donor lymphocytes after transplantation, rather than having been transmitted at the time of transplantation.
Practices to avoid donor-transmitted cancer mean that there are relatively few published reports of transmission. These limited reports do not cover the range of cancers for which decisions may be necessary, nor are case reports a reliable indicator of risk for all cancers of a given type. The decision to proceed with a given donor will therefore need to be informed by a combination of:
i. Reported cases of donor-derived cancer
ii. Observed lack of transmissions in the case of certain cancers (e.g. low grade, low stage prostate cancer)
iii. Known biology of cancers in non-immunosuppressed individuals (especially known recurrence rate)
iv. Known biology of de novo cancers in immunosuppressed hosts.
While it is inevitable that there will always be occasional cases of inadvertent cancer transmission, every effort should be made to identify past or current malignancy and to obtain as much information as possible to assess the risk of cancer transmission. This will inform (i) the decision as to whether the donation of any organ is safe, (ii) the risk-benefit assessment for each potentially suitable organ against individual recipient circumstances, and (iii) necessary steps to mitigate risk if a decision is made to proceed. Investigations should include:
Obtaining a full donor history, including details of any past cancer diagnosis and treatment and any other information relevant to assessing the risk of malignancy transmission based on current disease status
Checking jurisdictional cancer registry (see Appendix L)
Undertaking a careful physical examination at the time of donor workup and during the surgical retrieval procedure
Reviewing test results including those available as part of patient care or routinely undertaken for donor assessment, and any additional tests indicated for further evaluation of cancer risk, including imaging and biopsy
Seeking expert oncological and other advice, as required.
2.4.1 Summary recommendations for organ utilisation
Sections 2.4.3 to 2.4.7 of this document provide information on risks and recommendations for organ utilisation in the event of malignancy being detected in a potential donor. The estimated risks of donor-derived transmission associated with cancers of different sites are summarised in Table 2.7 below. Cancer types and stages associated with minimal-to-low risk of transmission and those associated with high-to-unacceptable risk are shown. For recommendations with respect to other cancer types and stages (including those associated with an intermediate risk of transmission), refer to the cancer site-specific section in this document. Recommendations for organ utilisation associated with each risk classification group are given in Table 2.8.
Table 2.7: Summary of risks associated with malignancies of different sites in the donor history or detected at retrieval.
Risk classification categories:
Minimal risk of transmission (<0.1%) – Likely to be acceptable for all organ types and recipients
Low risk of transmission (0.1% to <2%) – Likely to be acceptable for many organ types and recipients
High risk of transmission (≥10%) – May be acceptable in exceptional circumstances
Unacceptable risk – Use of organs is not recommended in any circumstance
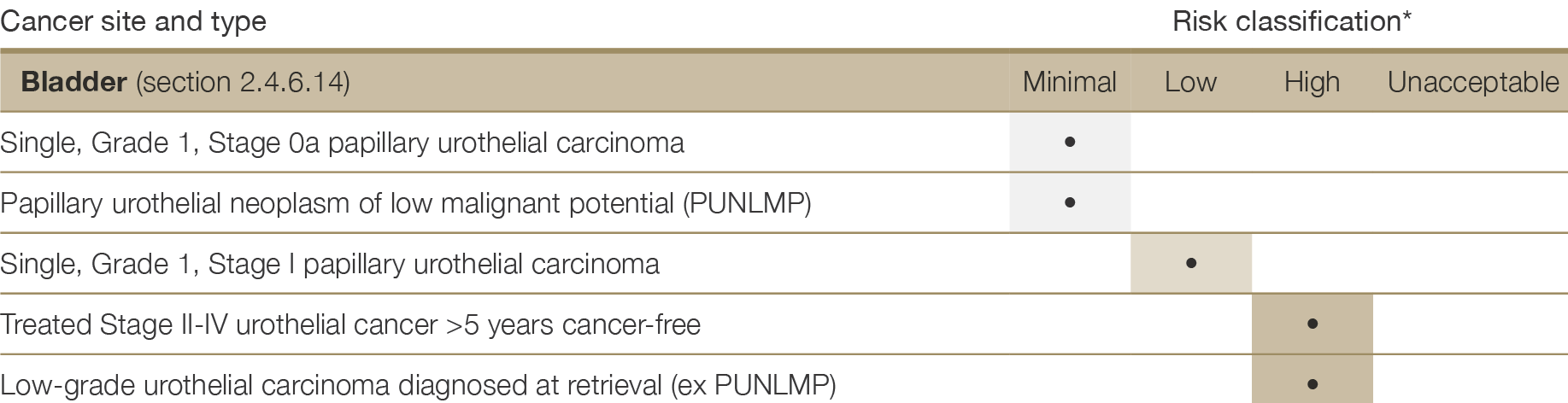
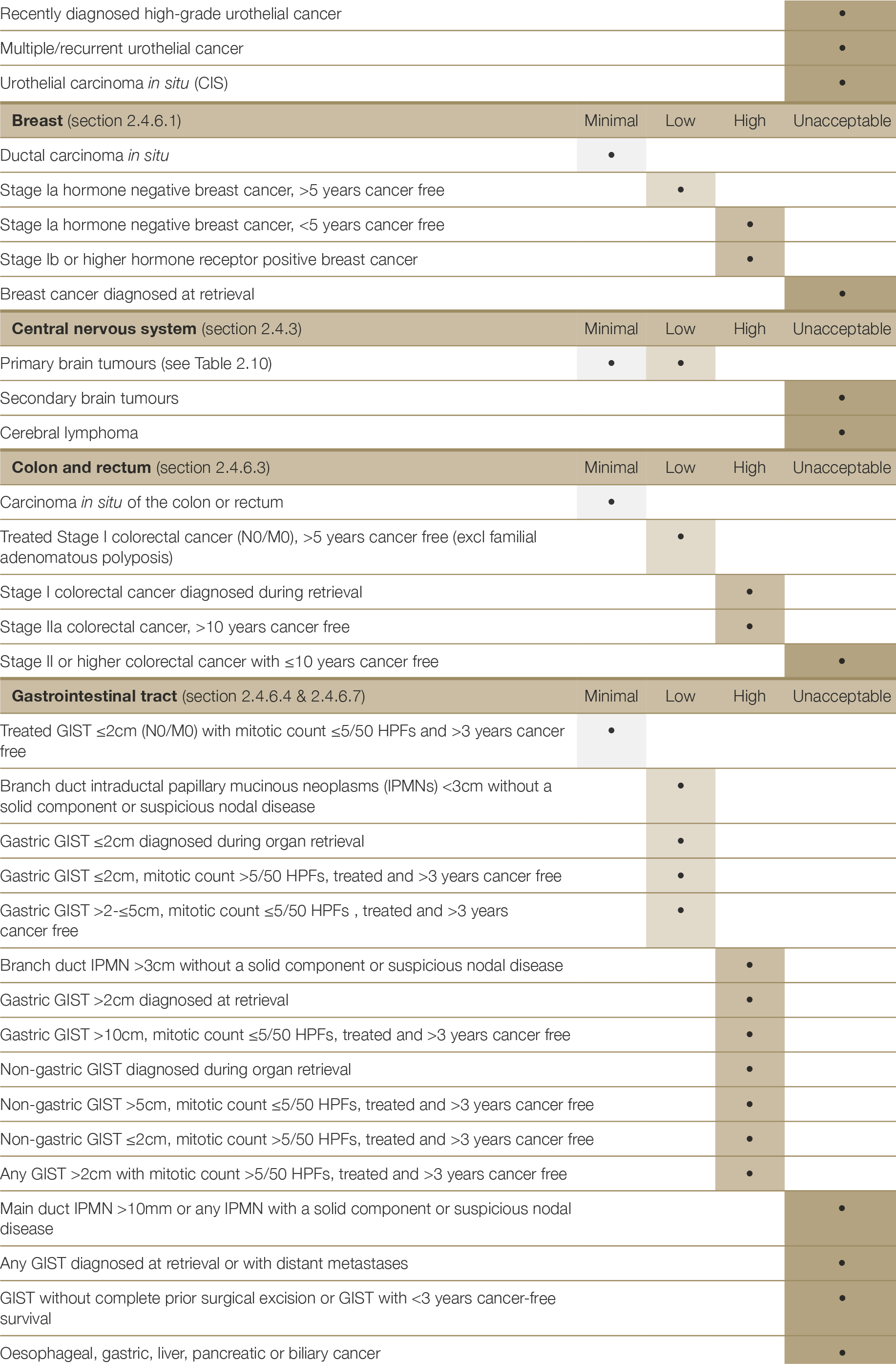
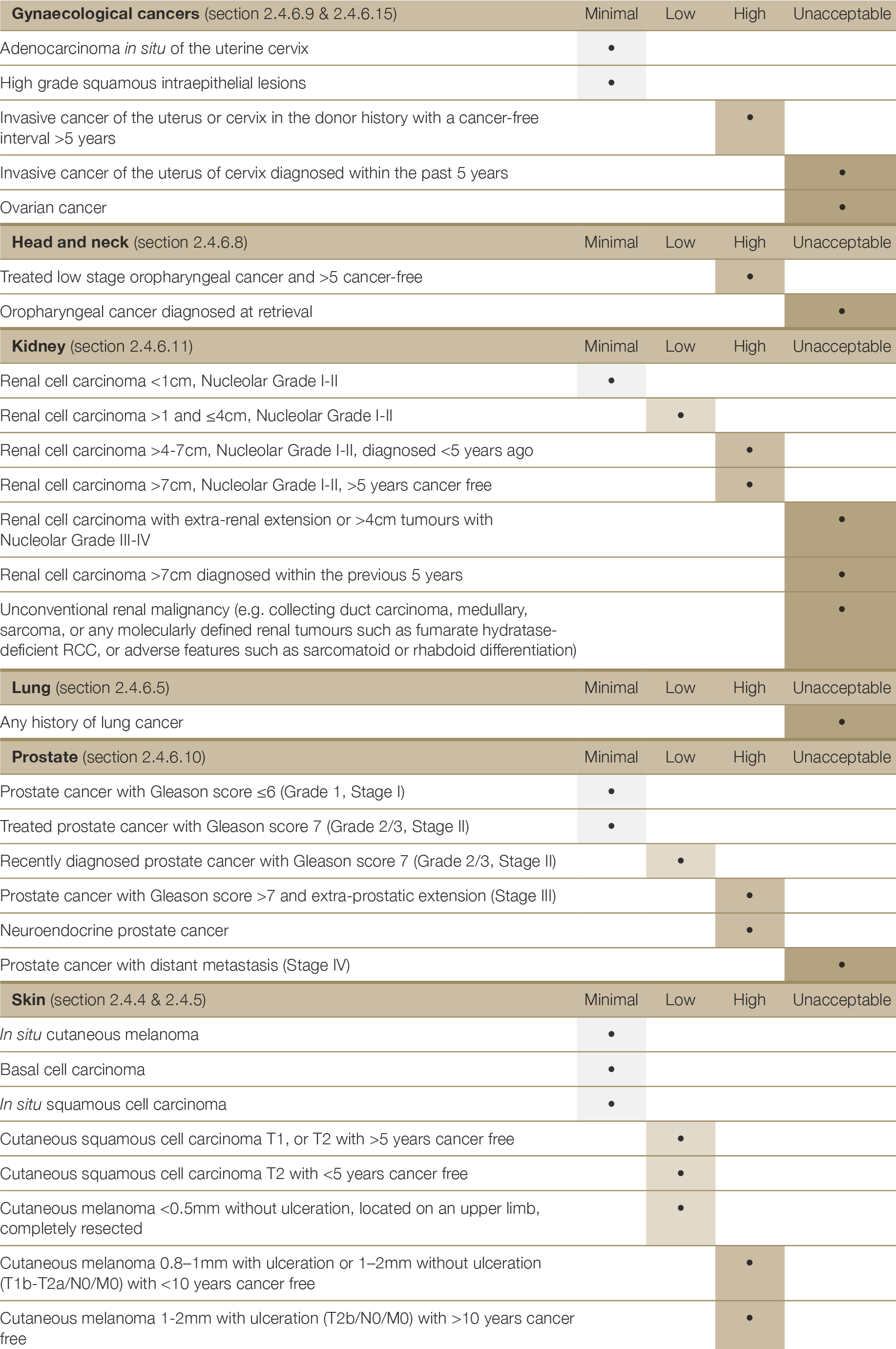
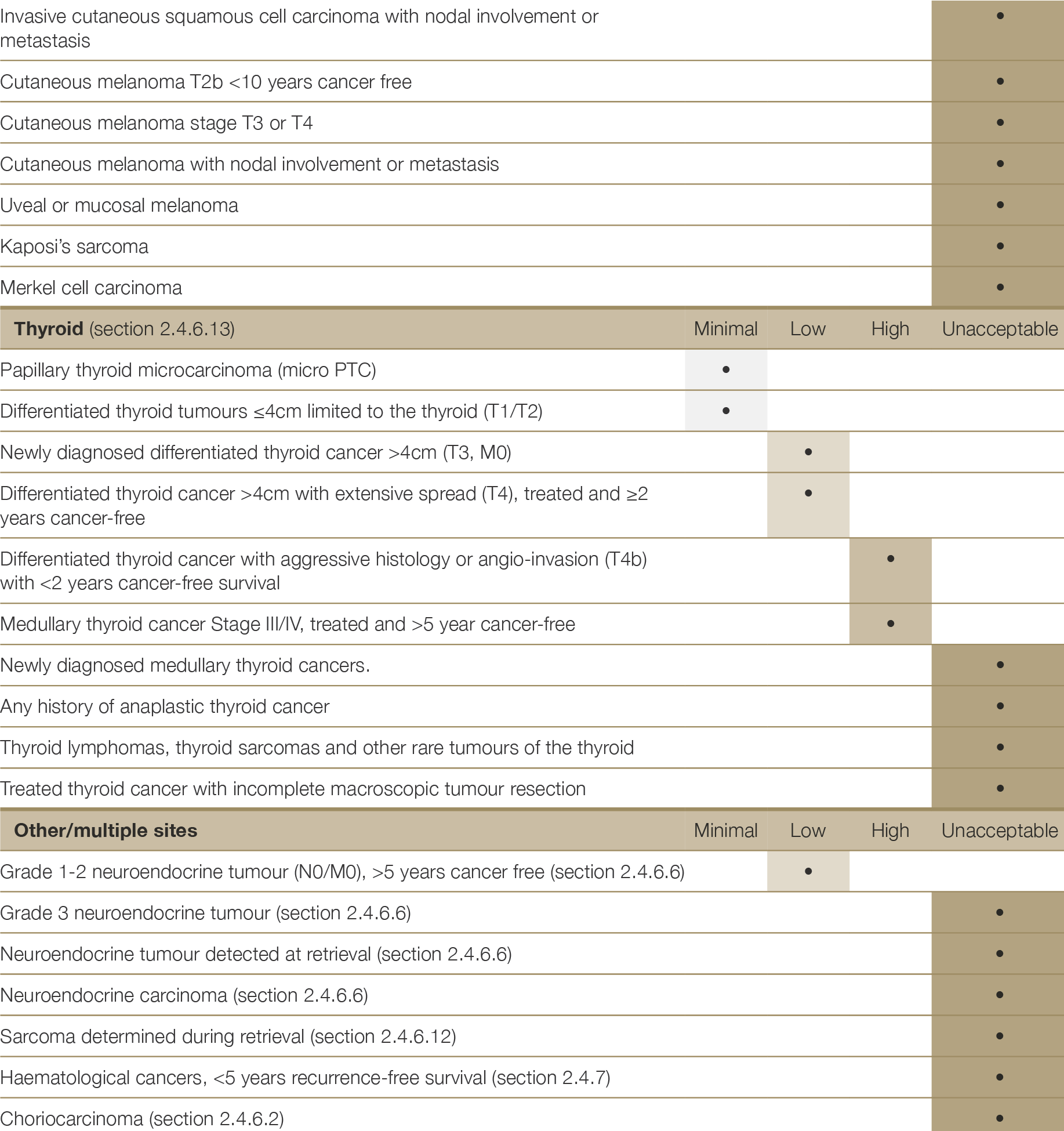
2.4.2 General recommendations for detecting malignancy in the donor and assessment of transmission risk
2.4.2.1 Donor history
At donor assessment, an interview is undertaken with the donor’s family by the donation specialist coordinator or other health professional. This includes questions about past history of cancer and removal of any skin lesions, participation in regular cancer screening (e.g. prostate examinations in male donors and regular breast examinations and/or pap smears in female donors) and other factors that may indicate a risk of cancer, including smoking or recent weight loss. Donor assessment may also include a history of genetic testing identifying a cancer predisposition mutation in the donor or their first-degree blood relatives (see Appendix K).
If there is a reported history of cancer, details should be sought about the type, when and where it was diagnosed, treatments (e.g. surgery, radiotherapy, chemotherapy) and follow up. Pathology reports of removed skin lesions are often able to be obtained from the donor’s general practitioner or pathology laboratories. Detailed information about a history of cancer should be obtained, including information from the patient’s oncologist and treatment centre, stage of cancer, and histopathology reports. Reports related to disease surveillance, including imaging, should also be obtained if possible.
Details of all enquiries made in relation to donor history of cancer should be documented and communicated to the transplant centres. Where investigations were undertaken in response to a history of cancer reported by family, but no information was found, this should also be communicated to the transplant centres.
2.4.2.2 Donor physical examination
Physical examination occurs at two steps of the organ retrieval pathway: the first by the donation specialist coordinator or other health professional at the time potential donor is being considered, and the second at the time of organ retrieval by the surgical team. The physical examination undertaken by the donor coordinator ideally occurs as soon as possible after the family interview, so that information obtained from the family can be verified at the physical assessment and the coordinator can seek further clarification from the family about unexpected findings (e.g. scars from prior surgery). The physical examination includes a careful assessment to detect scars, skin lesions and obvious lumps or masses. Large scars that may indicate prior excision of a melanoma are of more concern than pigmented skin lesions, which are difficult to precisely identify and unlikely to represent an undiagnosed melanoma. The donor coordinator is not expected to make a precise diagnosis but rather to identify unusual or abnormal findings and seek further expertise from dermatology specialists, as well as documenting and conveying any abnormal findings to transplant units. The DonateLife physical assessment guideline provides further detail on donor physical examination.
The second physical examination undertaken by the surgeon(s) at the time of retrieval may reveal unexpected clinically occult lesions such as bowel cancers, renal or liver tumours. Abnormal lesions identified at this time may require biopsy to further evaluate the risk of malignancy. Perinephric fat should be excised sufficiently to ensure that the kidney is fully perfused. Careful dissection at the renal hilum is not routinely recommended at the donor hospital, but careful palpation of the kidney to exclude any renal mass is recommended.
2.4.2.3 Laboratory and imaging results
Pathology and imaging test results may be a) available as part of prior patient assessment, b) obtained as part of routine donation workup, and/or c) obtained through specific additional testing (e.g. imaging and biopsy).
Investigations that were undertaken as part of patient care and for the purpose of cancer treatment prior to the donation process beginning should be reviewed as part of the donor assessment process. These may include blood tests, imaging studies, biopsies or other information. Hospital medical record information, pathology results, and imaging study reports should be reviewed. Sometimes, an absence of any abnormalities is reassuring information that can be used to make a risk determination – e.g. computed tomography (CT) chest imaging revealing a lack of abnormalities in potential lung donors who are older and/or who have a heavy smoking history. If abnormalities are found, further evaluation and investigations may be warranted (see below).
Standard donor workup blood tests include a full blood examination and liver function tests. Occasionally, these can be abnormal as a result of an underlying malignancy. In female donors of child-bearing age, testing for beta human chronic gonadotrophin hormone is recommended to detect metastatic choriocarcinoma, especially if the cause of death is unexplained intracerebral haemorrhage. In all potential donors, a routine chest x-ray should also be carefully reviewed for any features that may suggest underlying primary or secondary pulmonary malignancy, or that might indicate bone metastatic disease in ribs or spine.
Abdominal CT imaging for potential liver donors may be requested, particularly if there are risk factors for malignancy or for anatomical evaluation (e.g. candidate for split &/or multivisceral donation). In general, such requests should be made with a provisional acceptance of the liver pending an acceptable CT scan result.
Additional tests, such as imaging, blood tests and/or biopsy, may be appropriate for further evaluation of cancer risk in certain, specific circumstances. Some centre-based or state-based protocols recommend CT chest studies to (i) exclude lung malignancy in potential lung donors aged over 70 years and/or those with a >20 pack year history of smoking, (ii) further evaluate concerning abnormalities identified on prior chest x-ray, or (iii) lung donors who test positive for SARS-CoV-2 on PCR from the upper respiratory tract to assess for any sequelae of COVID-19 (see Appendix E). A CT Chest that has already been undertaken as part of patient assessment ealier in the admission will usually suffice.
Routine screening for tumour markers is not recommended, since false-positive results may lead to unnecessary loss of donation and transplantation opportunities. If there is a confirmed malignancy in the donor history and previous tumour marker results are available, appropriate tumour markers may be tested as guided by oncological advice.
Careful consideration must be given to protocols or individual requests for additional tests, weighing their utility against the impact that undertaking such tests may have. Potential negative impacts include resource use (ICU and hospital), extending the donation workup timeframe and potential loss of family consent for donation as a result, and the potential for adverse impact on other organs. Additional investigations also introduce the risk of incidental findings (e.g. benign lesions identified on imaging, elevated prostatic specific antigen levels in catheterised male donors) that pose no risk to recipients and for which further evaluation is not possible in the donation timeframe, resulting in loss of transplantation opportunities. Hence, ad hoc requests for additional screening tests should only be carried out where there is a sound clinical or epidemiological basis for proceeding.
2.4.2.4 Biopsy and histopathology
Where a mass suspected of being malignant is known from the donor history or is found during donor workup and cannot clearly be determined as benign radiologically, a biopsy should be taken as part of the donor workup or planned ahead and performed during organ retrieval. For any mass or lymphadenopathy suspected of malignancy discovered during organ retrieval, an urgent histopathological examination must be performed by cytological smear and/or frozen section before any organ is transplanted. Where possible, discuss the appropriate sampling type with the on-call histopathologist. If possible, the mass should be completely resected and examined, without sacrificing a graft suitable for transplantation. The pathologist should be provided with as much clinical information as possible. If, however, after pathological examination the possibility that a suspicious mass is malignant cannot be ruled out, then the donor should be declined unless there is a suitable recipient facing an imminent risk to life (who has given informed consent).
When a donor malignancy (primary tumour or metastasis) is identified shortly after organ retrieval (e.g. during the implantation procedure), all recipient centres involved must be alerted immediately. In cases where organs have already been transplanted and histology reveals a malignancy (e.g. incidental cancer in a lung lobe discarded due to size reduction), a full donor autopsy should be requested whenever possible to obtain detailed information about tumour origin and dissemination. This is not necessary in cases of small primary renal cell carcinoma found in one kidney, which would not preclude the transplantation of other organs.
While routine post-mortem examination has become an uncommon procedure in clinical medicine, if an autopsy is performed then the results should be followed-up by the donation service up as the autopsy may detect potentially transmissible disease.
2.4.2.5 Assessment of transmission risk
For donors with a known history of cancer or where cancer is detected during donor work-up or organ retrieval, in deciding whether to proceed with donation it is important to not only consider to the natural history of the cancer in the donor, but also the potential impact of transplanting tumour cells of this type into an immunosuppressed recipient. Advice may be sought from the patient’s treating oncologist or from oncologists and/or other specialists with particular expertise regarding the cancer under consideration (e.g. urologists who specialise in prostate cancer).
While the precise risk of transmission from donor to recipient of any given cancer is usually unknown, it is possible to broadly categorise the likely risk of transmission based on what is known about the cancer type and stage, its metastatic potential, and its patterns of recurrence in both the transplant and non-transplant setting (see Table 2.8). Newly diagnosed invasive cancer generally poses a higher risk of transmission than a history of treated invasive cancer followed by a significant disease-free interval. For potential donors with a confirmed history of cancer, the risk of transmission will be influenced by the treatment received, the length of the disease- free interval, and follow-up history.
Table 2.8: Risk categories for donor malignancy transmission (adapted from Nalesnik et al 2011). In all cases where the risk of donor malignancy transmission is non-standard, specific informed consent should be obtained from the recipient or their delegate prior to proceeding with transplantation.
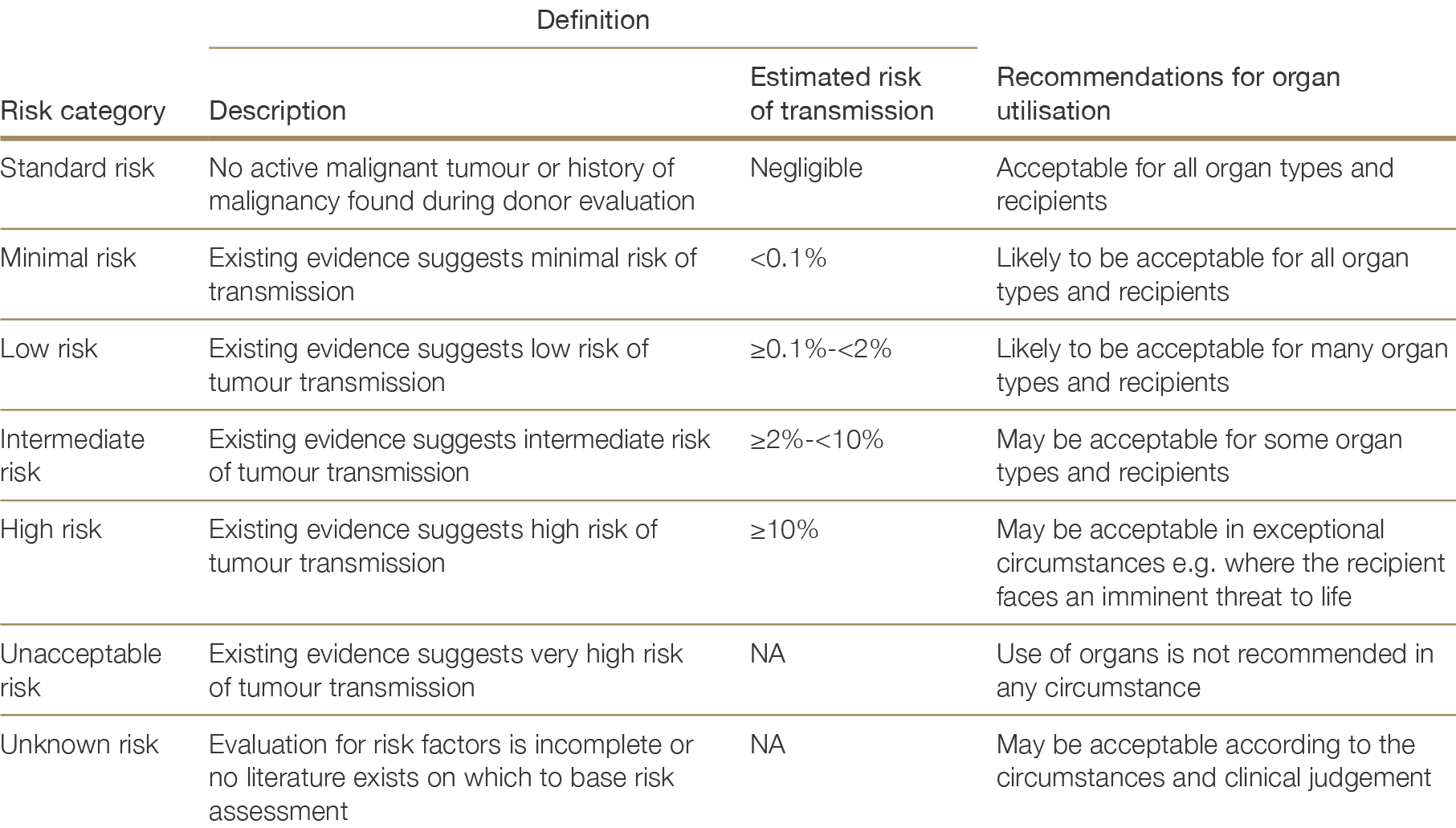
Organs from deceased donors with cancers categorised as minimal risk may be safely used for transplantation without particular restrictions. Organs from donors with low- to intermediate-risk cancers may also be acceptable and provide benefit for many recipients, depending on organ type and recipient circumstances. Thorough donor assessment can help to inform decision-making, as can advice from oncologists and other specialists.
When donors are at high risk of transmitting cancer, early medical advice regarding suitability is recommended and transplant units should be contacted to determine whether there are suitable recipients in whom the risk may be deemed acceptable, before proceeding with detailed donor assessment. In particular, liver and thoracic transplant services should be consulted to determine if there are any urgently listed patients who might be considered suitable recipients and may be willing to accept the high risk of cancer transmission.
In all circumstances, it is necessary to weigh the potential risks and benefits of proceeding with transplantation given the characteristics of a donor organ and the circumstances of a particular recipient. This tailored risk assessment will differ according to the donor and cancer risk, the organ being transplanted, the transplant urgency for a given recipient and – if the organ were to be declined – the likelihood of a subsequent more suitable offer being received in an acceptable time period.
Patients who are waitlisted for organ transplantation should receive education about the risks of transplantation, including that of possible donor-derived malignancy. When there is a specific risk associated with the donor organ(s) at the time of organ offer, specific informed consent should be obtained from the recipient or their guardian/delegated decision-maker if the recipient lacks competence (for example, due to young age or illness) prior to proceeding with transplantation.
2.4.2.6 Role of cancer registries
Cancer at most sites is a notifiable disease in Australia and New Zealand. Operational and governance arrangements vary amongst Australian states and territories, however the Australian Association of Cancer Registries encourages a standard approach to cancer data collection and management across Australia.
New Zealand and the Australian states and territories operate cancer registries that assemble information about new cases of cancer and cancer deaths, though the breadth of data collected can vary between jurisdictions (Table 2.9). All registries record location, morphological type, topography, diagnosis date, and basis of diagnosis. The registries are “case-based” data collections: each piece of information provided to the registry is considered in the context of other information about the same person and used to progressively create a complete picture of tumours for that person. Cases are generally identified by name, sex and date of birth. An important exception to mandatory cancer reporting is keratinocyte cancers (particularly squamous cell carcinomas).
In Australia, arrangements exist in some jurisdictions (Table 2.9) for DonateLife staff to query the state-based registry (either directly or indirectly) to obtain the history of a potential donor once appropriate consent for donation is obtained from the next of kin. However, information from the registry is limited to those cancers diagnosed or treated within that state or territory and is identified primarily by name and date of birth. In order to obtain a complete history, it is important to ascertain if the donor previously resided in a different state or territory, and/or may have been diagnosed or treated under a different name. Where the potential donor previously resided in another jurisdiction, DonateLife staff in that jurisdiction would need to be contacted to proceed with further enquires.
The registries forward an agreed list of data elements to the Australian Institute of Health and Welfare (AIHW) annually to a national repository—the Australian Cancer Database—for national reporting and disease monitoring. This data collection is not available for interrogation to obtain the malignancy history of a potential donor.
More information about Australian cancer registries is provided in Appendix L, including an overview of cancer-specific information available through each of the state-based registries. It should be noted that the information in Table 2.9 and Appendix L may change over time through continual improvement in data reporting and capture.
Table 2.9: Historic cancer data availability to DonateLife and Organ Donation New Zealand
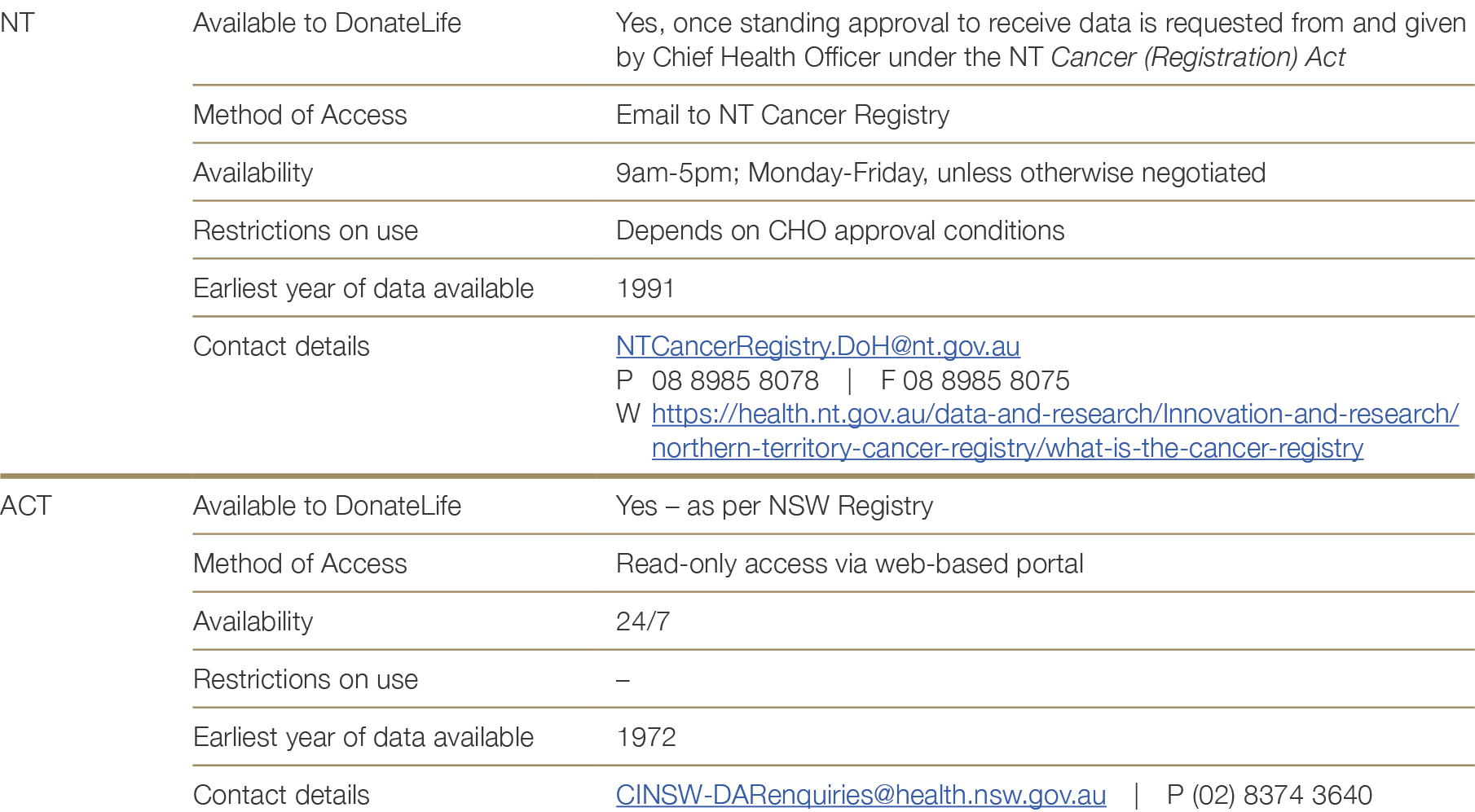
* A lot of information is captured on the pathology report, the inpatient notification (surgical) or on the radiotherapy/chemotherapy treatment notification, which may not be explicitly listed in a data field.
2.4.3 Central nervous system tumours
Donor assessment and eligibility
Primary solid central nervous system (CNS) tumours may occasionally lead to death in circumstances where organ donation is possible. Extracranial spread of brain tumours is rare, though there are reports of malignancy transmission to the recipients of organs from such donors.203,204,205,206,207,208,209,210,211,212203 Lefrancois N, Touraine JL, Cantarovich D et al. Transmission of medulloblastoma from cadaver donor to three organ transplant recipients. Transplant Proc, 1987; 19(1 pt 3):22-42. 204 Ruiz JC, Cotorruelo JG, Tudela V et al. Transmissino of glioblastoma multiforme to two kidney transplant recipients from the same donor in the absence of ventricular shunt. Transplantation, 1993; 55(3):682-3. 205 Colquhoun SD, Robert ME, Shaked A et al. Transmission of CNS malignancy by organ transplantation. Transplantation 1994; 57(6):970-4. 206 Jonas S, Bechstein WO, Lemmens HO et al. Liver gradt-transmitted glioblastoma multiforme. A case report and experience with 13 multiorgan donors suffering from primary cerebral neoplasia. Transplant Int, 1996;9(4):426-9. 207 Frank S, Müller J, Bonk C et al. Transmission of glioblastoma multiforme through liver transplantation. Lancet, 1998; 352(9121):31 Erratum in Lancet 1998; 352(9136):1316. 208 Armanios MY, Grossman SA, Yang SC et al. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: a case study and review of the literature. Neuro Oncol 2004; 6(3):259-63. 209 Zhao P, Strohl A, Gonzalez C et al. Donor transmission of pineoblastoma in a two-yr-old male recipient of a multivisceral transplant: a case report. Pediatr Transplant, 2012; 16(4):E110-14. 210 Val-Bernal F, Ruiz JC, Cotorruelo JG et al. Glioblastoma multiforme of donor origin after renal transplantation: report of a case. Human Pathol, 1993; 24(11):1256-9. 211 Morse JH, Turcotte JG, Merion RM et al. Development of a malignant tumor in a liver transplant graft procured from a donor with a cerebral neoplasm. Transplantation, 1990; 5(5):875-7. 212 Chen H, Shah AS, Girgis RE et al. Transmission of glioblastoma multiforme after bilalertal lung transplantation. J Clin Oncol, 2008; 26(19):3284-5. ×
Wherever possible, full histological characterisation of a CNS lesion should be accessed before any organ is retrieved. Where no histological diagnosis exists, organs from a donor with a CNS lesion should only be used in recipients whose probable waiting-list mortality justifies any extra risk, and only after fully informed consent has been given.
Assessment of transmission risk
Primary brain tumours
The World Health Organisation grades primary brain tumours from grade I to grade IV, based on biological behaviour and prognosis.213 Grade IV tumours are cytologically malignant and generally fatal, and this has been interpreted as representing the highest risk of donor-to-recipient transmission. However, a number of transplants from donors with grade IV tumours have been reported that did not result in transmission of malignancy to the recipient. A UK review of 448 recipients of organs from 177 donors with primary CNS tumours, including 23 donors with grade IV gliomas and 9 with medulloblastoma, found no evidence of tumour transmission over a minimum follow-up period of 5 years.214 A report of the Australian and New Zealand Organ Donor (ANZOD) registry found no transmission events from 46 donors, 9 with high-grade tumours, with organs transplanted into 153 recipients.215 A UNOS database report that included 642 recipients of organs from donors with CNS tumours between 2000 and 2005, including 175 recipients of organs from donors with high-grade tumours, identified a single donor with glioblastoma multiforme who transmitted disease to three recipients.216,217 A Czech report of 42 donors, 11 with high-grade tumours, found no transmission among 88 recipients followed for between 2 and 14 years.218213 Louis DN, Perry A, Reifenberger G, et al. The 2016 WHO classification of tumours of the central nervous system: a summary. Acta Neuropathol, 2016;131:803-820. ×214 Watson CJ, Roberts R, Wright KA, et al. How safe is it to transplant organs from deceased donors with primary intracranial malignancy? An analysis of UK Registry data. Am J Transplant, 2010;10(6):1437-44. ×215 Chui AK, Herbertt K, Wang LS, et al: Risk of tumor transmission in transplantation from donors with primary brain tumors: An Australian and New Zealand registry report. Transplant Proc, 1999; 31:1266–1267. ×216 Kauffman HM, Cherikh WS, McBride MA, et al: Deceased donors with a past history of malignancy: An Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation, 2007;84:272–274. 217 Armanios MY, Grossman SA, Yang SC et al. Transmission of glioblastoma multiforme following bilateral lung transplantation from an affected donor: case study and review of the literature. Neuro-oncology, 2004; 6(3): 259-263. ×218 Pokorna, E and Vitko S. The fate of recipients of organs from donors with diagnosis of primary brain tumor. Transpl Int, 2001;14(5):346-7. ×
Overall, UK and European guidelines estimate the risk of tumour transmission from WHO grade I and II tumours to be minimal (<0.1%), and the risk of transmission from grade III tumours to be low (0.1 to <2%). WHO grade IV tumours are estimated to have a low-to-intermediate risk of transmission. SaBTO in the UK estimate the risk of transmission of WHO grade IV tumours as 2.2%, although combined data from the registries in the UK, United States and Australia and New Zealand would indicate the risk of transmission is lower than this (<1 in 200). A larger evidence base is needed to definitively quantify the transmission risks associated with grade IV CNS tumours.
Interventions such as brain irradiation, chemotherapy, previous craniotomy and ventriculo-peritoneal shunt may increase the risk of transmission of CNS malignancy from donors to recipients, possibly through breaching the blood brain barrier and so facilitating tumour spread.219,220 There is considerable debate about the role and significance of such interventions, however, and it is difficult to differentiate between causality and coincidence— it may be that some interventions are more commonly employed in tumours that are more likely to spread.219 Buell JF, Trofe J, Sethuraman G et al. Donors with central nervous system malignancies: are they truly safe? Transplantation 2003; 76(2):340-3. 220 Hoffman HJ, Duffner PK. Extraneural metastases of central nervous system tumours. Cancer, 1985; 56(7 Suppl):1778-82. ×
There are case reports of tumour deposits identified around the peritoneal end of a ventriculoperitoneal shunt,221 though the pattern of metastases is similar in those with and without shunts. The estimated increase in risk of extracranial metastasis attributable to the presence of a cerebrospinal fluid shunt is less than 1%.222 The presence of a shunt should not contraindicate donation, provided that there is meticulous examination of the shunt tract at the time of retrieval surgery.223 Similarly, prior craniotomy or biopsy should not contraindicate donation, however at the time of the retrieval procedure there should be a close examination of the craniotomy site and cervical nodes.223 During the evaluation of a donor with a CNS tumour, a thorough thoracic and abdominal exploration with visualisation and palpation of organs should be performed.223221 Jamjoom ZA, Jamjoom AB, Sulaiman AH, et al. Systemic metastasis of medulloblastoma through ventriculoperitoneal shunt: report of a case and critical analysis of the literature. Surg Neurol, 1993;40(5):403-10. ×222 Warrens AN, Birch R, Collett D et al. Advising potential recipients on the use of organs from donors with primary central nervous system tumours. Transplantation, 2012; 93(4):348-353. ×223 Cavaliere R and Schiff D. Donor transmission of primary brain tumours: a neurooncologic perspective. Transplantation Reviews, 2004;18(4):204-213. ×223 Cavaliere R and Schiff D. Donor transmission of primary brain tumours: a neurooncologic perspective. Transplantation Reviews, 2004;18(4):204-213. ×223 Cavaliere R and Schiff D. Donor transmission of primary brain tumours: a neurooncologic perspective. Transplantation Reviews, 2004;18(4):204-213. ×
In summary, primary CNS tumours are not automatic contraindications to organ donation (with the exception of primary cerebral lymphoma – see below). In each case where a primary CNS tumour has been identified, the risk of not receiving a transplant should be weighed against the risk of transmission of donor malignancy. Based on reported data, the absolute risk of transmission of a primary CNS tumour is likely to be low, even with a high-grade malignancy. Craniotomy or other breach of the blood brain barrier does not contraindicate donation, though a ventriculo-systemic shunt may slightly increase the risk of transmission.222 Informed consent of the recipient is required, with the (derived) risk estimates shown in Table 2.10 provided to inform the recipient’s decision. More information on the possible transmission risks associated with specific CNS tumour types can be found in the European Guide to the Quality and Safety of Organs for Transplantation.224222 Warrens AN, Birch R, Collett D et al. Advising potential recipients on the use of organs from donors with primary central nervous system tumours. Transplantation, 2012; 93(4):348-353. ×224 Chapter 9: Risk of Transmission of neoplastic diseases. Guide to the Quality and Safety of Organs for Transplantation (7th ed.). European Directorate for the Quality of Medicines & Health Care, Council of Europe, 2018. ×
Secondary brain tumours and cerebral lymphoma
Donors with cerebral lymphoma or secondary CNS tumours are considered an unacceptable risk for organ donation. Secondary malignancy in this context refers to metastatic cancer that has spread to the brain from a primary site elsewhere in the body. There is a risk that donors with brain metastases may be misdiagnosed as having a primary brain tumour or intracerebral haemorrhage. Metastases should therefore be considered for any patient with a history of cancer presenting with a non-traumatic cerebral haemorrhage and, if suspected, donation should not proceed.
Table 2.10: Recommendations on the use of organs from donors with primary CNS tumours, by tumour site. Derived from SaBTO202 and the 2016 WHO classification of tumours of the central nervous system.225 For a list ordered by risk category, see Appendix M.202 Transplantation of Organs from Deceased Donors with Cancer or a History of Cancer. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2014. ×225 Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. World Health Organisation Histological Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer, 2016, France. ×

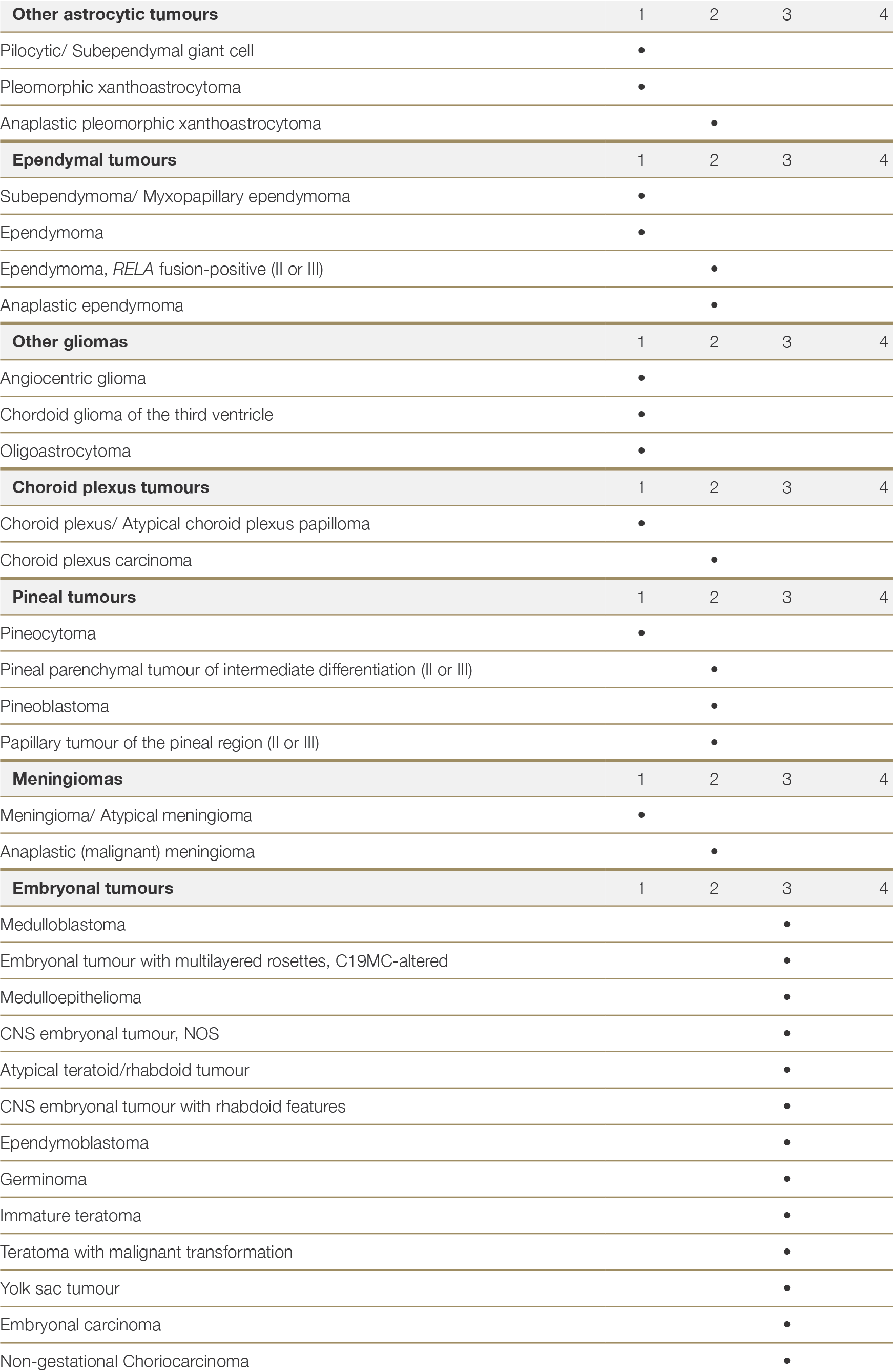
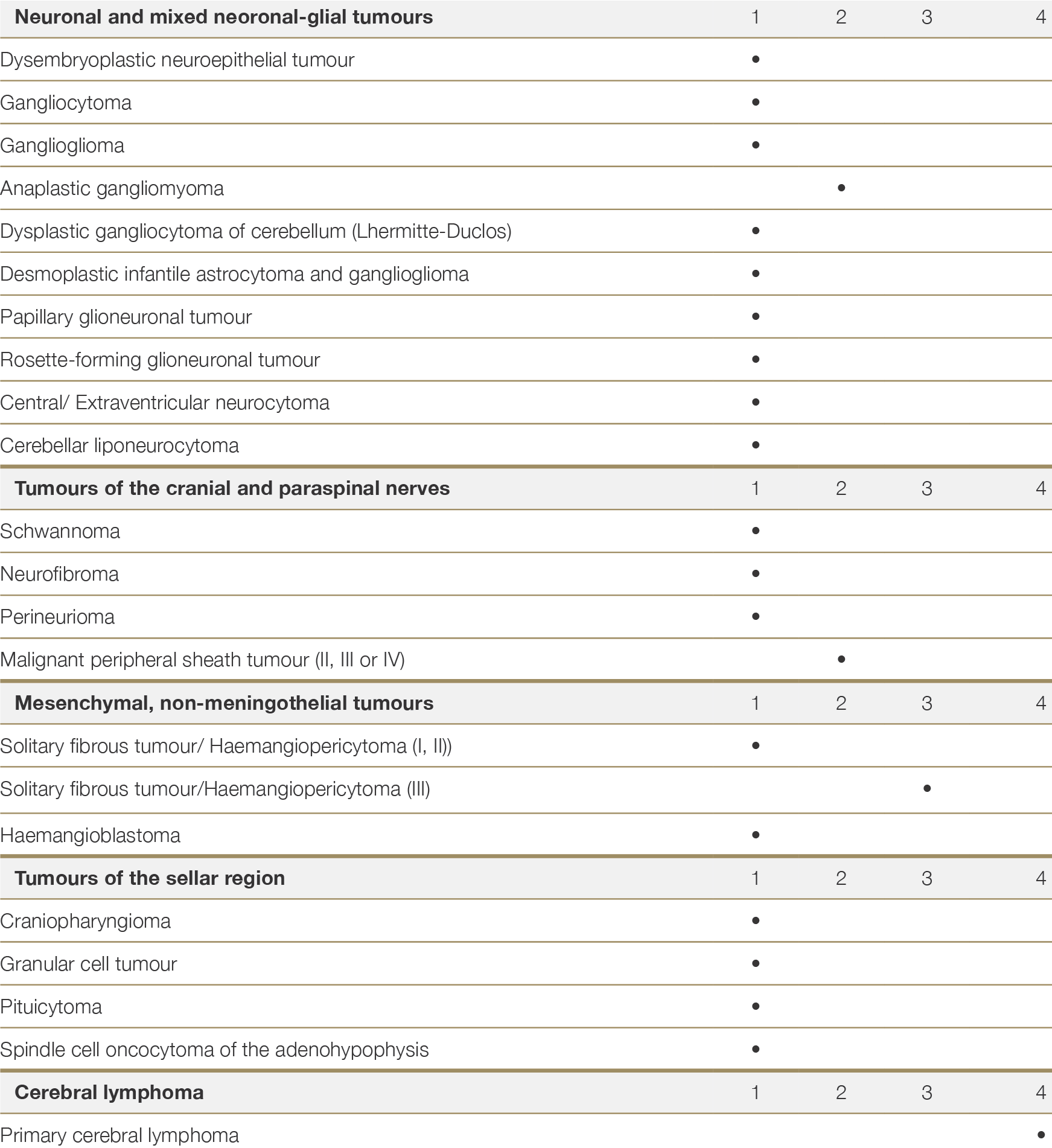
Risk classification categories:
1 Minimal risk of transmission (<0.1%) – Likely to be acceptable for all organ types and recipients
2 Low risk of transmission (0.1% to <2%) – Likely to be acceptable for many organ types and recipients
3 Low-to-intermediate risk of transmissiona – consider on a case-by-case basis
4 Unacceptable risk
a Best available evidence suggests that the risk of transmission from donor to recipient in the case of grade IV CNS tumours is ≤2%146a No reference text available.×146 HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2017. Kirby Institute, University of New South Wales, Sydney, Australia, 2017. ×
2.4.4 Melanoma
Melanoma is the third-most commonly diagnosed invasive cancer in Australia and New Zealand.226,227 There are numerous reports of donor-derived transmission of cutaneous melanoma in the international literature, mostly where tumour diagnosis was missed in the donor.216,228,229,230,231,232,233 Due to its high population prevalence, risk of non-detection, and tendency for early micrometastasis, invasive melanoma is the most commonly transmitted tumour type, accounting for approximately 30% of all reports of donor-transmitted cancers.234,235 Transmission of melanoma from donor to recipient is also associated with a high recipient mortality rate – the IPITTR estimates this at 60%.230226 New Cancer Registrations 2018. New Zealand Ministry of Health. Published online 17 December 2020 (https://www.health.govt. nz/publication/new-cancer-registrations-2018). 227 Australia’s Leading Cancers, incidence and mortality by age and sex groups, 1982 to 2020. Cancer data in Australia. Australian Institute of Health and Welfare, Web Report (https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-rankings-data-visualisation, last updated 13 Nov 2020). ×216 Kauffman HM, Cherikh WS, McBride MA, et al: Deceased donors with a past history of malignancy: An Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation, 2007;84:272–274. 228 Green M, Covington S, Taranto S et al. Donor-derived transmission events in 2013: a report of the Organ Procurement Transplant Network Ad Hoc Disease Transmission Advisory Committee. Transplantation, 2015 February;99(2):282-7. 229 Birkeland SA and HH Storm. Risk for tumor and other disease transmission by transplantation: a population-based study of unrecognized malignancies and other diseases in organ donors. Transplantation, 2002;74(10):1409-13. 230 Buell JF, Beebe TM, Trofe J et al. Donor transmitted malignancies. Ann Transplant 2004; 91(1):53-6. 231 Chen KT, Olszanski A, Farma JM. Donor transmission of melanoma following renal transplant. Case Rep Transplant 2012;2012:764019. 232 Cankovic M, Linden MD, Zarbo RJ. Use of microsatellite analysis in detection of tumor lineage as a cause of death in a liver transplant patient. Arch Pathol Lab Med 2006;130(4):529-32. 233 Morris-Stiff G, Steel A, Savage P et al. Transmission of donor melanoma to multiple organ transplant recipients. Am J Transplant 2004;4(3):444-6. ×234 Penn I. Transmission of cancer from organ donors. Nefrologia, 1995; 15(3):205-302. 235 Zwald FO, Christenson LJ, Billingsley EM et al. Melanoma in solid organ transplant recipients. Am J Transplant, 2010; 10(5): 1297- 304. ×230 Buell JF, Beebe TM, Trofe J et al. Donor transmitted malignancies. Ann Transplant 2004; 91(1):53-6. ×
The exact risk of cutaneous melanoma transmission is strongly related to Breslow thickness and melanoma stage at diagnosis and treatment.236 In situ cutaneous melanoma is, by definition, non-invasive and poses minimal risk of donor-derived transmission, given that in situ melanoma is not associated with metastatic risk. In a case series of 140 transplants with grafts from donors diagnosed with melanoma, including a mix of in situ and invasive melanoma, only one case of transmission was reported.216236 Dicker TJ, Kavanagh GM, Herd RM et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. The British Journal of Dermatology, 1999, 140(2):249-254. ×216 Kauffman HM, Cherikh WS, McBride MA, et al: Deceased donors with a past history of malignancy: An Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation, 2007;84:272–274. ×
Invasive cutaneous melanoma, in contrast, may recur even after many years of disease-free survival and is associated with an intermediate-to-unacceptable risk of transmission. True recurrence rates are difficult to estimate as people may die of competing cause before recurrence is detected. Melanoma-specific survival rates in people with primary cutaneous melanoma may therefore be used as a guide to the potential risks of transmission. Using the latest staging (AJCC 8th edition,237) individuals with melanomas < 0.8mm without ulceration are classified as T1a. In non-immunosuppressed individuals, the 10-year mortality risk associated with T1a melanoma is reported to be between 2-7%.238,239 189,190 However, population-based studies have assessed 20-year melanoma-specific survival in patients with a melanoma less than 0.5mm and reported risks of melanoma death of less than 2%.238,240 For melanomas of thickness >0.8mm, risks of recurrance maybe greater than 10%, depending on features and site.239 In addition to tumour thickness, factors including ulceration, presence of mitoses, head and neck site, nodular or acral type and male sex have an adverse impact on recurrence rates.241 The Melanoma Institute Australia (MIA) provide a risk calculator for estimating the recurrence risk associated with thin melanomas up to 1mm in thickness (https://www.melanomarisk.org.au/ThinMelForm).237,238237 Gershenwald JE, Scolyer RA, Hess KR et al Melanoma Staging: evidence-based changes in the American Joint Committee on Cancer eight edition cancer staging manual. Ca: A cancer journal for clinicians 2017;67(6) 474-492 ×238 Green AC, Baade P, Coory M, Aitken JF, Smithers M Population-Based 20-Year Survival Among People Diagnosed With Thin Melanomas in Queensland, Australia. J Clin Oncol 2012;30(13):1462-1467 ×239 Lo SN, Scolyer RA and Thompson JF. Long-term survival of patients with thin (T1) cutaneous melanomas: a Breslow thickness cut point of 0.8 mm separates higher-risk and lower-risk tumors. Ann Surg Oncol, 2018; 25(4): 894-902. Baade PD, Whiteman DC, Janda M, et al. Long-term deaths from melanoma according to tumor thickness at diagnosis. Int J Cancer 2020;147(5):1391-1396 ×238 Green AC, Baade P, Coory M, Aitken JF, Smithers M Population-Based 20-Year Survival Among People Diagnosed With Thin Melanomas in Queensland, Australia. J Clin Oncol 2012;30(13):1462-1467 ×240 Baade PD, Whiteman DC, Janda M, et al. Long-term deaths from melanoma according to tumor thickness at diagnosis. Int J Cancer 2020;147(5):1391-1396×239 Lo SN, Scolyer RA and Thompson JF. Long-term survival of patients with thin (T1) cutaneous melanomas: a Breslow thickness cut point of 0.8 mm separates higher-risk and lower-risk tumors. Ann Surg Oncol, 2018; 25(4): 894-902. Baade PD, Whiteman DC, Janda M, et al. Long-term deaths from melanoma according to tumor thickness at diagnosis. Int J Cancer 2020;147(5):1391-1396 ×241 Isaksson K, Mikiver R, Eriksson H et al. Survival in 31 670 patients with thin melanomas: a Swedish population-based study. Br J Dermatol, 2021;184:60-67. ×237 Gershenwald JE, Scolyer RA, Hess KR et al Melanoma Staging: evidence-based changes in the American Joint Committee on Cancer eight edition cancer staging manual. Ca: A cancer journal for clinicians 2017;67(6) 474-492 238 Green AC, Baade P, Coory M, Aitken JF, Smithers M Population-Based 20-Year Survival Among People Diagnosed With Thin Melanomas in Queensland, Australia. J Clin Oncol 2012;30(13):1462-1467 ×
Melanoma cells may spread to distant sites in the early stages of cancer progression and can stay dormant and clinically undetectable for decades after resection of the primary tumour.242 Transplantation of an organ with dormant melanoma micrometastases into an immunosuppressed host may lead to metastatic growth in the recipient,243,244,245with generally poor survival outcomes.246,247 Use of life saving organs might still be considered in certain circumstances, however, after discussion with a melanoma specialist and with informed consent of the recipient.242 Crowly NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg, 1990 Aug;212(2):173-7. ×243 Piérard-Franchimont C, Hermanns-Lê T, Delvenne P, Piérard G. Dormancy of growth-stunted malignant melanoma: sustainable and smoldering patterns. Oncol Rev, 2014; 8(2):252. 244 Tseng WW, Fadaki N, Leong SP. Metastatic tumor dormancy in cutaneous melanoma: does surgery induce escape? Cancers, 2011; 3(1):730-46. Linde N, Fluegen G, Aguirre-Ghiso JA. The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv Cancer Res, 2016, 132:45-71. 245 Linde N, Fluegen G, Aguirre-Ghiso JA. The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv Cancer Res, 2016, 132:45-71.×246 Benoni H, Eloranta S, Ekbom A, Wilczek H, Smedby KE. Survival among solid organ transplant recipients diagnosed with cancer compared to nontransplanted cancer patients—A nationwide study. Int J Cancer. 2019;146(3):682–91. 247 Robbins HA, Clarke CA, Arron ST et al. Melanoma Risk and Survival among Organ Transplant Recipients. J Invest Derm, 2015; 135(11):2657-2665. ×
Uveal and mucosal melanoma pose an unacceptable risk to donation, given a high risk of undetected micrometastases, regardless of the length of disease-free survival.248,249,250 Cutaneous melanoma with a history of nodal involvement or distant metastases also poses an unacceptable risk.248 Kaliki S and CL Shields. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond) 2017;31(2):241-57. 249 Carvajal RD, Schwartz GK, Tezel T et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017;101(1):38-44. Altieri L, Eguchi M, Peng DH, Cockburn M. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol, 2019; 81(1):136-142. 250 Altieri L, Eguchi M, Peng DH, Cockburn M. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol, 2019; 81(1):136-142.×
Donors with a history of invasive melanoma should only be accepted in cases where sufficient information is available to make a determination of risk status. If sufficient information is not available to determine risk status (e.g. histology report unavailable or short duration of cancer follow-up time), donation should only be considered in exceptional circumstances.
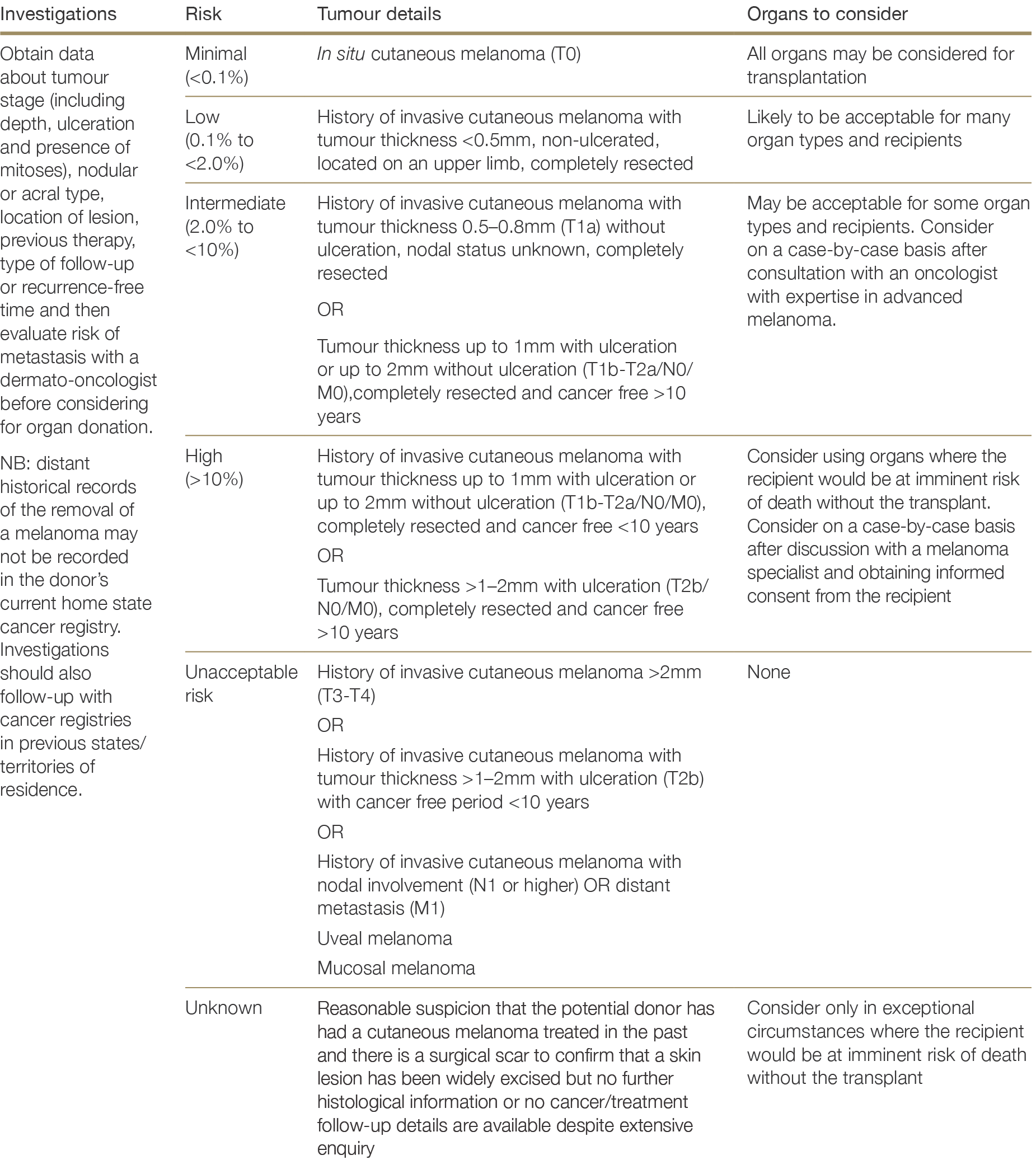
2.4.5 Non-melanoma skin cancers
Non-metastatic keratinocyte cancers (basal cell and squamous cell carcinoma of the skin) are the most common malignancy encountered in donors and are not considered a contraindication to donation. All basal cell carcinoma (BCC) and the majority of squamous cell carcinoma (SCC) are considered minimal-to-low risk of transmission: a UNOS database report including 776 recipients of organs from donors with BCC and SCC of the skin reported no incidence of disease transmission.216216 Kauffman HM, Cherikh WS, McBride MA, et al: Deceased donors with a past history of malignancy: An Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation, 2007;84:272–274. ×
Potential donors with a history of a large SCC (>2cm) and fewer than 5 years of recurrence free follow-up, however, pose a higher risk of transmission and should be considered on a case-by-case basis.251 For donors with more than 5 years of recurrence-free follow-up, the risk of SCC transmission is low, regardless of tumour size.251 Karia PS, Morgan FC, Califano JA, Schmults CD. Comparison of tumour classifications for cutaneous squamous cell carcinoma of the head and neck in the 7th vs 8th Edition of the AJCC Cancer Staging Manual. JAMA Dermatol, 2018; 154(2):175-181. ×
Other types of cancers manifesting on the skin, such as Kaposi sarcoma (see Section 2.3.2.7), spindle cell carcinoma and Merkel cell carcinoma, pose an unacceptable risk to transplantation.
Figure 2.4: Decision flow chart for potential donors with a history non-melanoma skin cancer
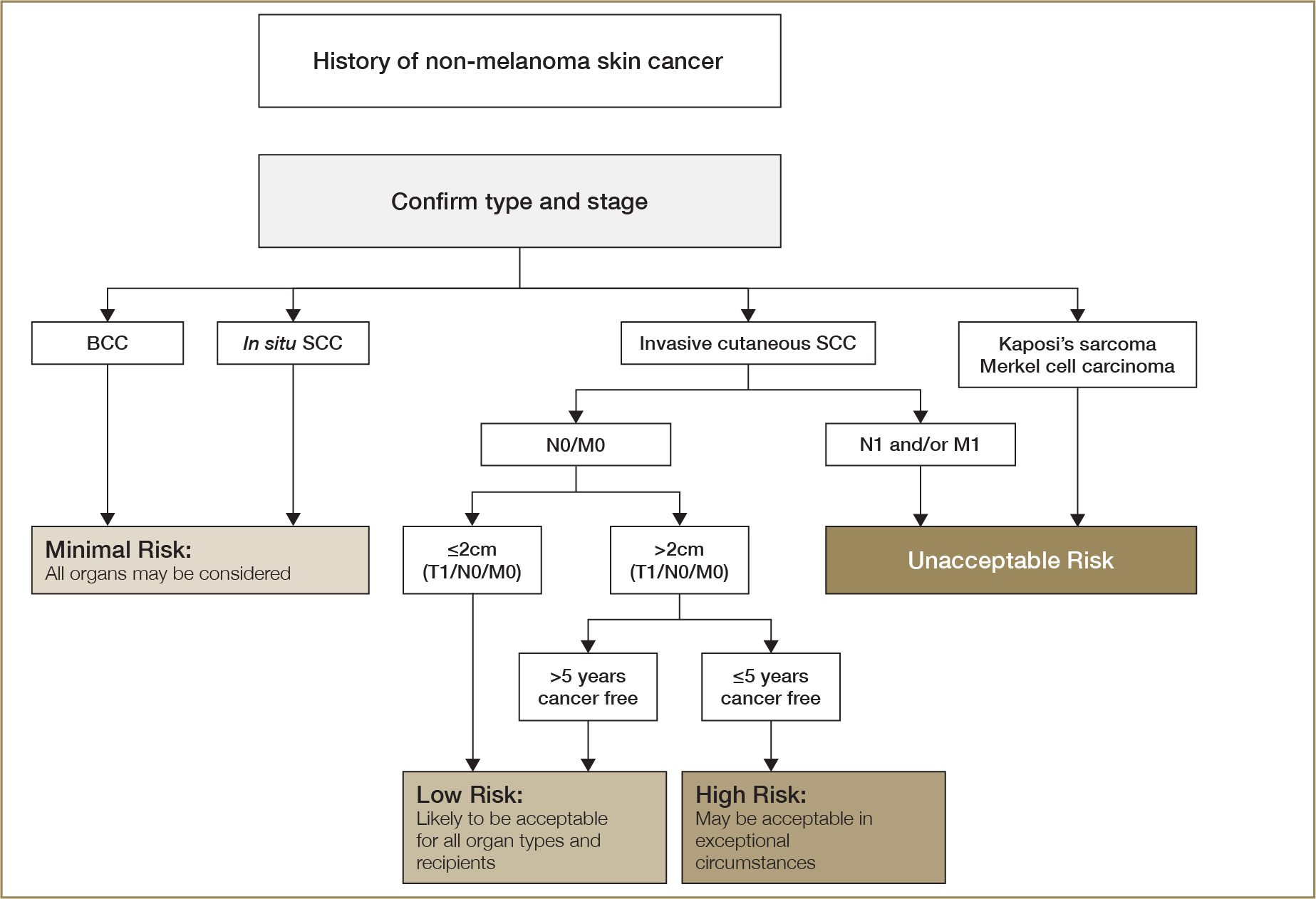
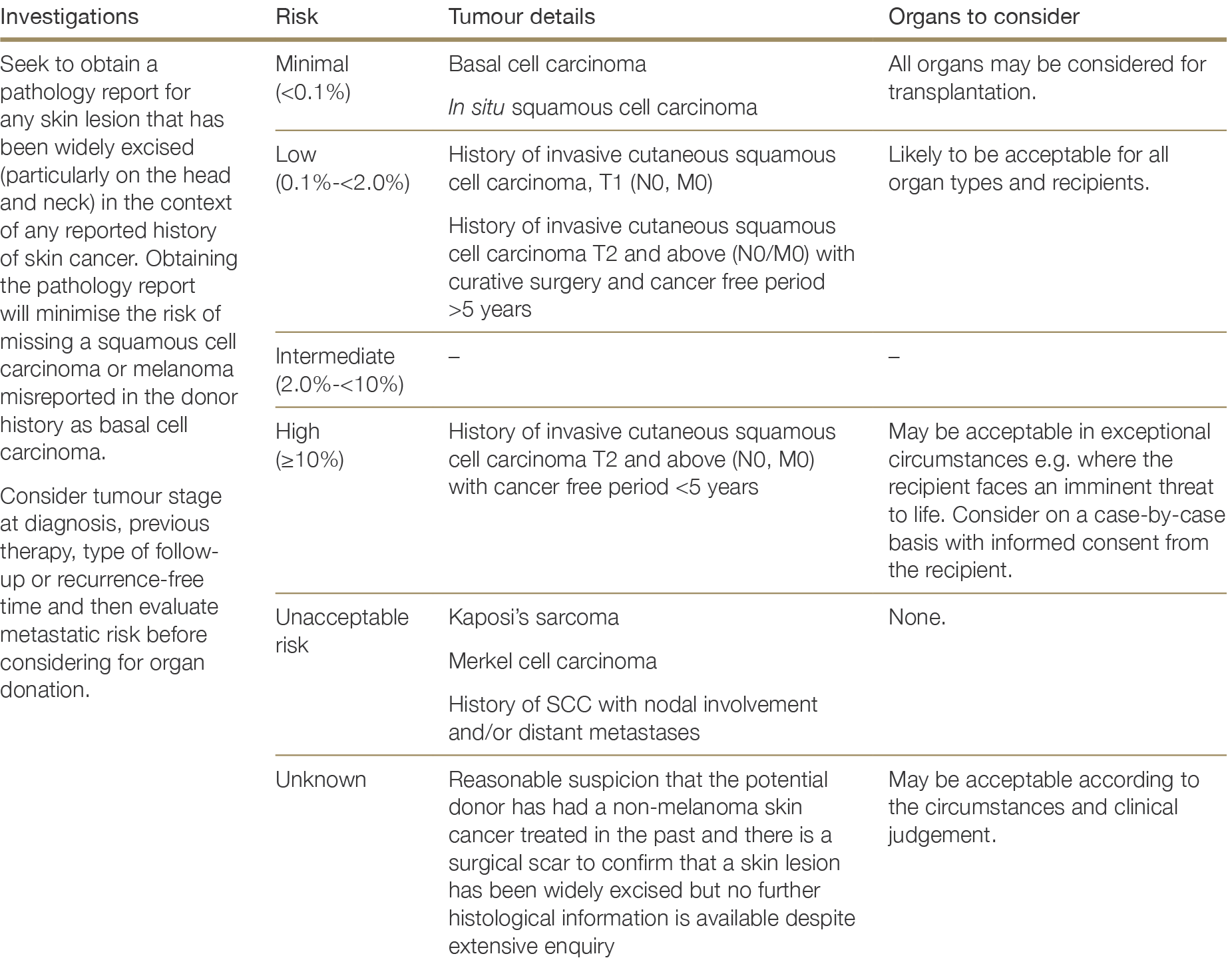
2.4.6 Solid organ tumours
2.4.6.1 Breast cancer
Given the potential for late recurrence and metastasis, organs from donors with a history of invasive breast cancer should only be considered where specific criteria indicative a low risk of transmission are met.252,253 Organs from donors with a history of Stage I (T1A, node-negative) hormone receptor-negative breast cancer may be considered where there has been full treatment and complete remission with follow-up for >5 years.254 All other invasive breast cancer is considered high-risk of transmission, regardless of the duration of recurrence-free survival. For hormone positive breast cancer, the cumulative risk of recurrence at 20-25 years post-treatment is high, even for Stage 1 disease.252,253 For Stage I oestrogen-receptor positive breast cancer without nodal involvement, there is a 13% risk of recurrence at 20 years.252 Given the late recurrence associated with hormone-positive breast cancer, donors with a history of cancer of this type have a high risk of malignancy transmission.252 Pan H, Gray R, Braybrooke J et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 yeasrs. N Engl J Med; 377(19):1836-46. 253 Gonzalez-Angulo AM, Litton JK, Broglio KR et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumours 1cm or smaller. J Clin Oncol, 2009 (27):5700-6. ×254 Balkenhol MCA, Vreuls W, Wauters CAP et al. Histological subtypes in triple negative breast cancer are associated with specific information on survival. Annals Diag Pathol, 2020 (46): 151490. Wang K, Zhu G, Shi Y et al. Long-term survival differences between T1-2 invasive lobular breast cancer and corresponding ductal carcinoma after breast conserving surgery: a propensity-scored matched longitudinal cohort study. Clinical Breast Cancer, 2018; 19(1): 101-15. ×252 Pan H, Gray R, Braybrooke J et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 yeasrs. N Engl J Med; 377(19):1836-46. 253 Gonzalez-Angulo AM, Litton JK, Broglio KR et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumours 1cm or smaller. J Clin Oncol, 2009 (27):5700-6. ×252 Pan H, Gray R, Braybrooke J et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 yeasrs. N Engl J Med; 377(19):1836-46. ×
While lobular breast cancer is usually of greater clinical concern, in terms of the risk of distant recurrence the risks are similar between lobular and ductal carcinoma of the breast.255 Hence, in the context of assessing risk of transmission in the case of a Stage I breast cancer with >5 years recurrence free survival, it is reasonable to group lobular and ductal carcinoma together.255 Wang K, Zhu G, Shi Y et al. Long-term survival differences between T1-2 invasive lobular breast cancer and corresponding ductal carcinoma after breast conserving surgery: a propensity-scored matched longitudinal cohort study. Clinical Breast Cancer, 2018; 19(1): 101-15.×
Where donors have a known history of invasive breast cancer but critical information on cancer pathology, treatment history, and/or receptor status are unavailable, donation should only be considered under exceptional circumstances for recipients facing an imminent threat to life. Invasive breast cancer diagnosed during retrieval poses an unacceptable risk to potential transplant recipients.
It is important to verify breast cancer history as reported by donor proxy/next of kin, to rule out the possibility of misreporting. Every effort should be made to establish that breast cancer was present as reported before excluding a potential donor from further investigation.
Ductal carcinoma in situ poses minimal risk of donor-derived transmission and does not preclude organ donation.
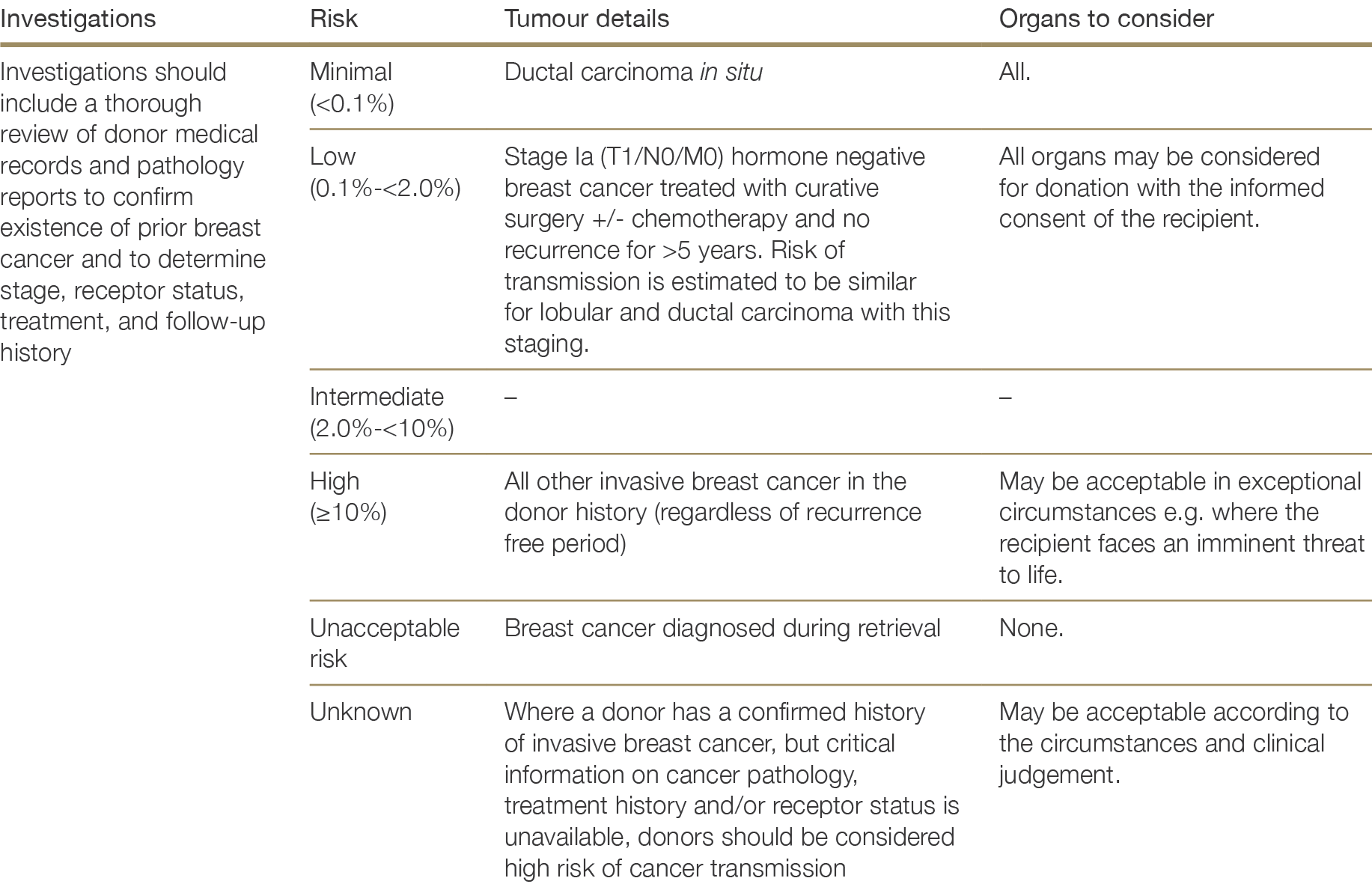
2.4.6.2 Choriocarcinoma
Choriocarcinoma of any stage is considered an unacceptable risk for organ transplantation. Choriocarcinoma is a highly aggressive, malignant trophoblastic cancer arising after hydatidiform mole, pregnancy, ectopic pregnancy, miscarriage or termination. Cases of undetected choriocarcinoma in donors resulting in multiple transmissions demonstrate the malignancy of choriocarcinoma in immunosuppressed recipients.230,256 Female donors of child-bearing potential should have their blood levels of beta human chorionic gonadotrophin hormone tested to detect metastatic choriocarcinoma, especially if the cause of death is unexplained intracerebral haemorrhage.230 Buell JF, Beebe TM, Trofe J et al. Donor transmitted malignancies. Ann Transplant 2004; 91(1):53-6. 256 Braun-Parvez L, Charlin E, Caillard S et al. Gestatinoal choriocarcinoma transmission following multiorgan donation. Am J Transplant, 2010; 10(11):2541-6. ×
2.4.6.3 Colorectal cancer
Based on an exceedingly low risk of nodal or metastatic disease associated with Stage I (T1, node-negative) colorectal cancers in the general population, a 2003 US consensus conference endorsed the use of such donors in certain circumstances.257 In donors with Stage I familial adenomatous polyposis, however, the pancreas should be excluded from transplantation due to the increased risk of cancers in the duodenum, although some other organs may be acceptable according to the circumstances and clinical judgement. In donors with familial adenomatous polyposis where the colon remains in situ, given the immense number of polyps it would be impossible to rule out the presence of a malignant lesion. The risk of malignancy transmission in this circumstance would therefore most accurately be classified as unknown.257 Feng s, Buell JF, Chari S, et al. Tumours and transplantation: The 2003 Third Annual ASTS State-of-the-Art Winter Symposium. Am J Transplant, 2003;3:1481-1487. ×
Stage II or higher colorectal cancer diagnosed at retrieval, or in the donor history with a cancer-free duration ≤10 years, poses an unacceptable risk of transmission to recipients. It is important that pathology reports are available to definitely establish cancer stage before proceeding with transplantation. Retrieval surgeons should carefully examine all intra-abdominal and intra-thoracic structures for suspicious lesions.
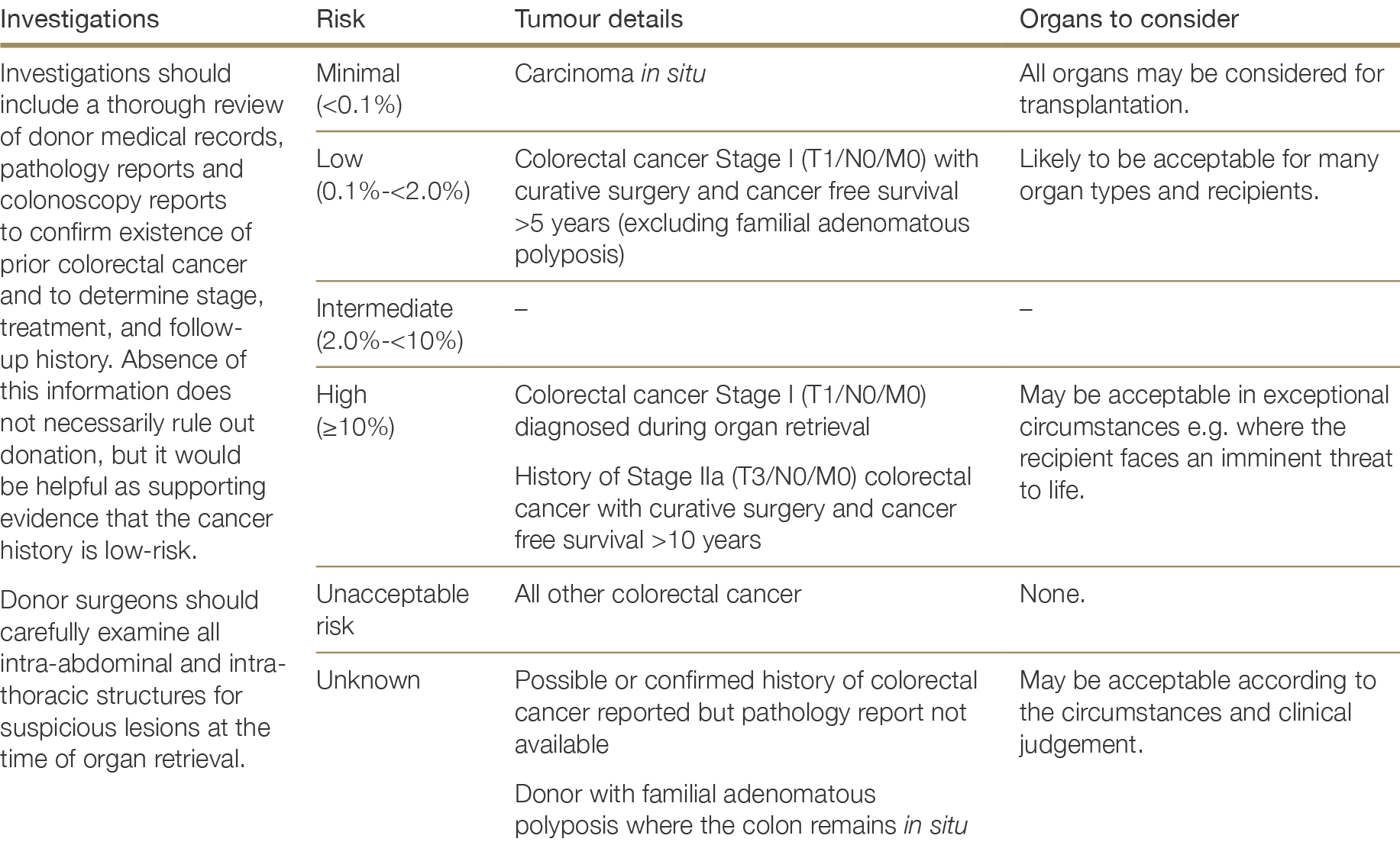
2.4.6.4 Gastrointestinal stromal tumour
Gastrointestinal stromal tumours (GIST) account for 4-5% of soft tissue sarcomas and 1-2% of all gastrointestinal malignancies.258,259 Incidence of clinically diagnosed GIST is relatively low (an estimated 14 cases per million per year in Australia260 and approximately 10-20 cases per million per year in Europe261); however, small, subclinical GISTs are common in adults.262,263,264 The median age of people diagnosed with GIST is 60-65 years; paediatric GIST is a rare and clinically distinct subset of the disease.265 GISTs can involve almost any segment of the gastrointestinal tract but most commonly occur in the stomach (60%), jejunum and ileum (30%), duodenum (4-5%), or rectum (4%).261258 Bessen T, Caughey GE, Shakib S et al. A population-based study of soft tissue sarcoma incidence and survival in Australia: An analysis of 26 970 cases. Cancer Epidemiology, 2019; 63: 101590. 259 Demetri GD, von Mehren M, Antonescu CR et al. NCCN Task Force Report: update on the management of patients with gastrointestinal stromal tumours. J Natl Compr Canc Netw, 2010; 8:S1-41. Parameswaran R, Roberts RH, Brown WA et al. Surgery for gastrointestinal stromal tumours in Australia and New Zealand: results from a bi-national audit. ANZ J Surg, 2017; 87:220-223. ×260 Parameswaran R, Roberts RH, Brown WA et al. Surgery for gastrointestinal stromal tumours in Australia and New Zealand: results from a bi-national audit. ANZ J Surg, 2017; 87:220-223.×261 Miettinen M and Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol, 2006; 23(2):70-83. ×262 Nilsson B, Bümming P, Meis-Kindblom JM et al. Gastrointetinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era – a population-based study in Western Sweden. Cancer, 2005; 103:821-829. 263 Liegl-Atzwanger B, Fletcher JA, Fletcher CDM. Gastrointestinal stromal tumour. Virch Arch, 2010; 456:111-27. 264 Miettinen M and Lasota J. Gastrointestinal stromal tumours. Gastroenterol Clin North Am, 2013; 42(2):399-415. Casali PG, Abecassis N, Bauer S et al. Gastrointestinal stromal tumours: ESMA-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2018; 29(Suppl 4): iv68-iv78. ×265 Casali PG, Abecassis N, Bauer S et al. Gastrointestinal stromal tumours: ESMA-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2018; 29(Suppl 4): iv68-iv78.×261 Miettinen M and Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol, 2006; 23(2):70-83. ×
The median disease-free survival after GIST resection is approximately 3 years.266 The main prognostic factors are: (i) tumour localisation (site and spread) (ii) mitotic index, (iii) tumour size, and (iv) tumour rupture before or during surgery.266,267 Gastric GISTs have a more favourable prognosis and lower risk of metastases than GIST occurring at other primary sites, such as the small intestine.261 The metastatic potential of GISTs exists on a spectrum from small, inactive tumours to larger, mitotically active tumours;268 metastasis, where it occurs, is typically to the abdominal cavity or liver and may occur many years after treatment of the primary tumour.261,264,269266 Rutkowski P, Nowecki ZI, Michej W et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol, 2007; 14(7):2018-27. ×266 Rutkowski P, Nowecki ZI, Michej W et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol, 2007; 14(7):2018-27. 267 ESMO/European Sarcoma Network Working Group; Gastrointestinal stromal tumours: clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2014;25(Suppl 3):iii21-6. ×261 Miettinen M and Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol, 2006; 23(2):70-83. ×268 Parameswaran R, Roberts RH, Brown WA et a. Surgery for gastrointestinal stromal tumours in Australia and New Zealand: results from a bi-national audit. ANZ J Surg, 2017;87:220-223. ×261 Miettinen M and Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol, 2006; 23(2):70-83. 264 Miettinen M and Lasota J. Gastrointestinal stromal tumours. Gastroenterol Clin North Am, 2013; 42(2):399-415. Casali PG, Abecassis N, Bauer S et al. Gastrointestinal stromal tumours: ESMA-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2018; 29(Suppl 4): iv68-iv78. 269 DeMatteo RP, Lewis JL, Leung D et al. Two hundred gastrointestinal stromal tumors. Ann Surg, 2000; 231(1):51. ×
European guidelines state that donors with a history of gastric or duodenal GIST <2cm with mitotic index ≤5 per 50 high power fields (HPFs) have a low risk of metastases and may be acceptable for organ donation with low-to-intermediate risk of transmission.224 A case series from Italy of five GISTs diagnosed during donor retrieval, all under 2cm and with mitotic index ≤5/50 HPFs, reported no evidence of transmission after a minimum of 18 months follow up from the three organs transplanted (two kidneys from a single donor and a liver from a second donor).270 The Miettinen risk criteria define GISTs ≤2 cm as having zero risk of progressive disease over long term follow up, with gastric GISTS up to 5cm associated with a very low risk of progressive disease.264224 Chapter 9: Risk of Transmission of neoplastic diseases. Guide to the Quality and Safety of Organs for Transplantation (7th ed.). European Directorate for the Quality of Medicines & Health Care, Council of Europe, 2018. ×270 Novelli L, Messerini L, Caporalini C et al. Gastrointestinal stromal tumour diagnosed during donor procurement: The experience of a single institution and review of the literature. Med Sci Tech, 2017: 58:62-66 ×264 Miettinen M and Lasota J. Gastrointestinal stromal tumours. Gastroenterol Clin North Am, 2013; 42(2):399-415. Casali PG, Abecassis N, Bauer S et al. Gastrointestinal stromal tumours: ESMA-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2018; 29(Suppl 4): iv68-iv78. ×
GIST from primary sites other than the stomach that are >2cm and/or have a mitotic count >5/50 HPFs are likely to be associated with a higher risk of malignancy transmission, based on reported relapse rates on long-term follow-up of people with GIST.264,266,269,271 The exact risk of transmission will also depend on the treatment received, follow-up time and duration of recurrence free survival. A disease-free period of more than 3 years is likely to indicate lower risk of transmission.266264 Miettinen M and Lasota J. Gastrointestinal stromal tumours. Gastroenterol Clin North Am, 2013; 42(2):399-415. Casali PG, Abecassis N, Bauer S et al. Gastrointestinal stromal tumours: ESMA-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2018; 29(Suppl 4): iv68-iv78. 266 Rutkowski P, Nowecki ZI, Michej W et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol, 2007; 14(7):2018-27. 269 DeMatteo RP, Lewis JL, Leung D et al. Two hundred gastrointestinal stromal tumors. Ann Surg, 2000; 231(1):51. 271 Mandrioli M, Mastrangelo L, Masetti et al. Characterization of malignant gastrointestinal stromal tumors – a single center experience. J Gastrointest Oncol 2017; 8(6):1037-1045. ×266 Rutkowski P, Nowecki ZI, Michej W et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol, 2007; 14(7):2018-27. ×
In the context of GIST discovered during retrieval, the degree of risk is related to where the tumour is located, its size, and its mitotic count. As it is unlikely to be possible to obtain biopsy results and mitotic index in the timeframe required for organ transplantation, in this circumstance all known information about the tumour should be considered (i.e. size, location) and weighed against the risks to the recipient in the absence of transplantation. Gastric GISTS ≤2cm diagnosed at the time of retrieval are likely to be low risk, although minimal data are available on which to base recommendations.
Figure 2.5: Decision flow chart for potential donors with a history of gastrointestinal stromal tumour (GIST) or GIST detected at the time of organ retrieval
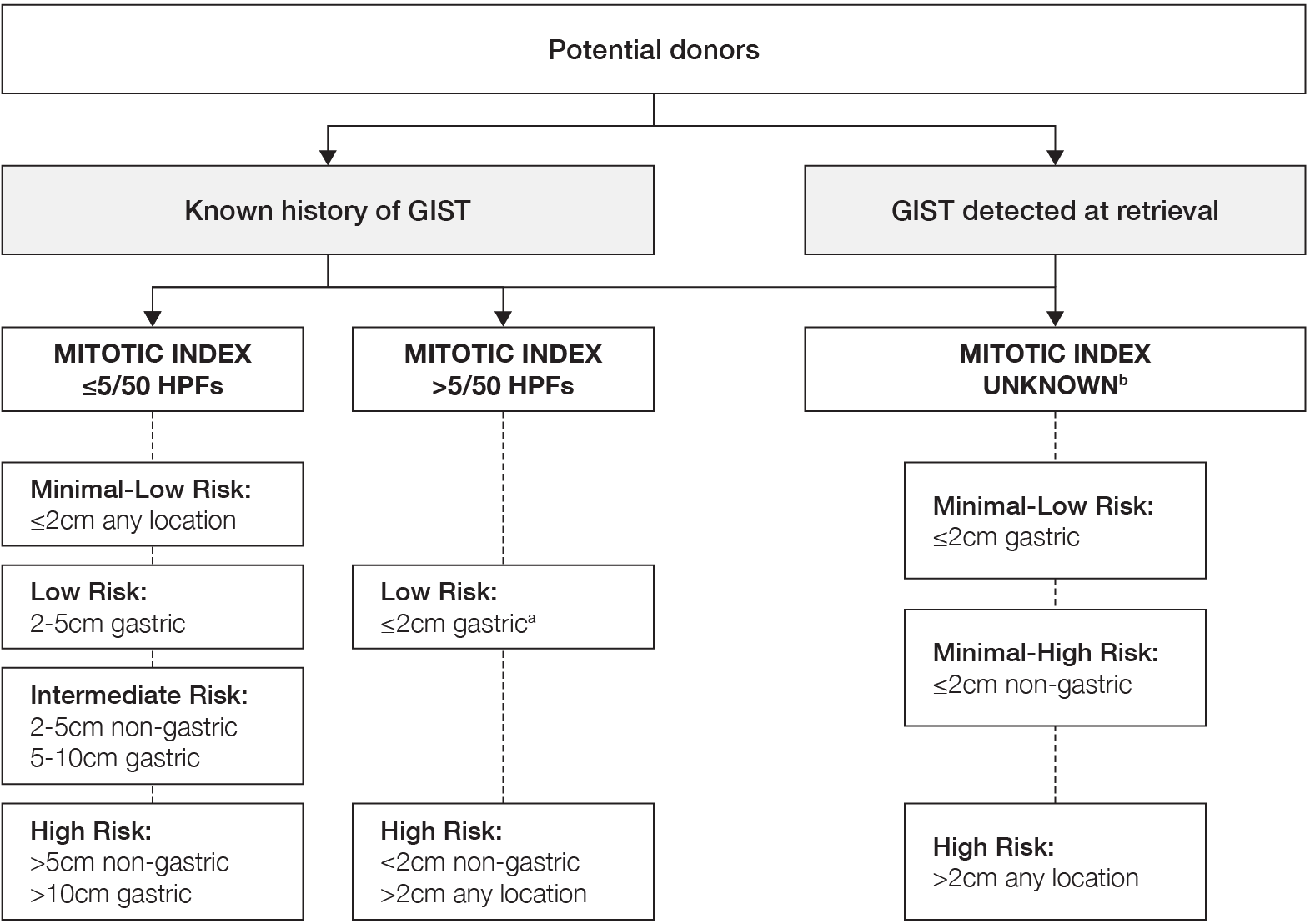
a Minimal data available on which to base risk assessmenta No reference text available.×
b Where mitotic index is not available, all known information about the tumour should be considered and weighed against the risks to the recipient in the absence of transplantationb No reference text available.×
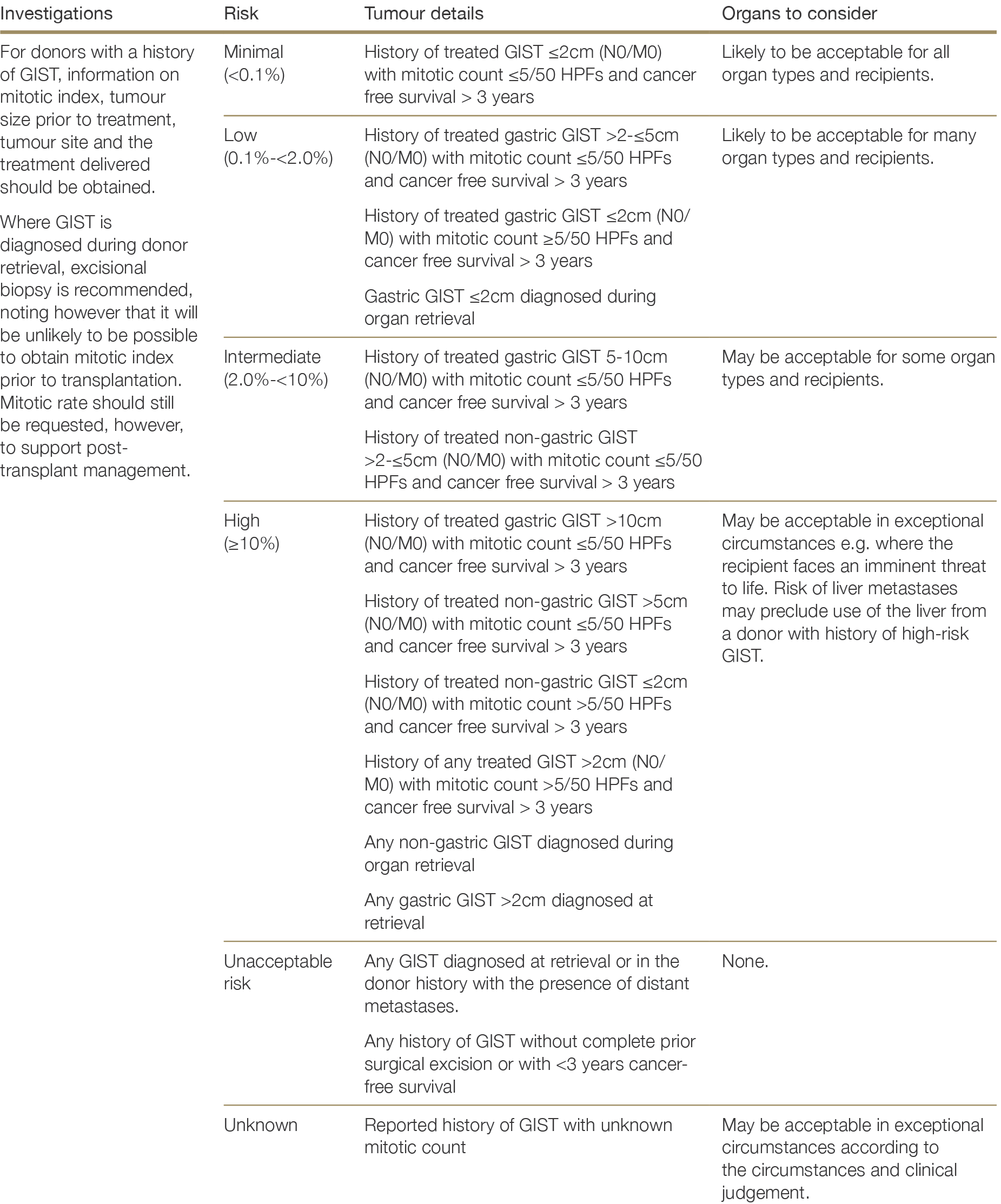
2.4.6.5 Lung cancer
A history of lung cancer of any stage poses an unacceptable risk to organ transplantation. Registry and case reports of transmission of occult donor lung cancer by kidney transplantation, with generally fatal outcomes in recipients, are indicative of very aggressive behaviour of donor-transmitted lung cancers.272,273,274272 Desai R, Collett D, Watson CJ et al. Cancer transmission from organ donors – unavoidable by low risk. Transplantation 2012; 94(12):1200-7. 273 Forbes GB, Goggin MJ, Dische FE et al. Accidental transplantation of bronchial carcinoma from a cadaver donor to two recipients of renal allografts. J Clin Pathol, 1981;34(2):109-15. 274 Göbel H, Gloy J, Neumann J et al. Donor-derived small cell lung carcinoma in a transplanted kidney. Transplantation 2007; 84(6):800-2. ×
Lung adenocarcinoma in situ (AIS) has a low risk of distant metastases and a low risk of recurrence, and therefore theoretically may be associated with a lower risk of transmission from organ donors to recipients. However, a definitive diagnosis of AIS is difficult to determine, as lesions may be multifocal and it is not possible to guarantee that there are no parts of the lesion with an invasive component. Theoretically – if a definitive diagnosis of AIS could be made – use of non-lung organs might be considered, recognising a small but real risk of disease transmission. In practice, donors with a diagnosis of AIS are unlikely to be suitable for donation of any organs.
Benign pulmonary nodules – such as hamartomas and papillomas – are relatively common especially after 45 years of age, hence it is important to distinguish between benign tumours in the lung and lung cancer in the donor. Donor assessment should include smoking history and appropriate imaging (CT) when indicated on the basis of an abnormality noted on chest x-ray or in the donor history, with review by a radiologist +/- respiratory physician. If available, any prior chest imaging should be reviewed for comparison. Lung bronchoscopy of potential lung donors is commonly deployed in all jurisdictions (where possible) and would permit visualisations of any endobronchial lesions.
Pulmonary hamartomas, which are common benign lesions, can be confidently diagnosed on CT. Other lesions may need to be assessed intraoperatively and a frozen section taken, especially in the context of significant smoking history or any suspicious signs on chest CT.
2.4.6.6 Neuroendocrine neoplasms
Neuroendocrine neoplasms mainly arise in gastrointestinal, lung or pancreatic tissue, but can be detected anywhere and may occur in multiple sites throughout the body. Although neuroendocrine neoplasms are rare, the rate of diagnosis of neuroendocrine neoplasms in Australia has doubled since the early 1980s and they are one of the most common incidental findings on organ retrieval.275 Neuroendocrine neoplasms are divided based on histological differences into well-differentiated neuroendocrine tumours (NETs), poorly differentiated neuroendocrine carcinomas (NECs), and mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs).276 Well-differentiated NETs are further divided into Grades 1 (low), 2 (intermediate), and 3 (high) based on mitotic rate and Ki-67 proliferation index; NECs are considered poorly differentiated and high-grade by definition and may be of small-cell or large-cell type.277275 Cancer data in Australia, web report, Australian Institute of Health and Welfare, 13 Nov 2020 (Cat. No: CAN 122) (available at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary) ×276 Rindi G, Klimstra DS, Abedi‐Ardekani B et al A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018; 31; 1770–1786. ×277 Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology, 2020; 76(2):182-188. ×
There have been multiple reports of donor-derived transmission of undetected high-grade NETs and small-cell NECs from liver and kidney donors, resulting in explant and/or death.278,279,280 NETs and NECs detected during organ retrieval pose an unacceptable risk for organ transplantation, given the known metastatic potential of these cancers and the likelihood of undetected micrometastases224 There may be certain circumstances of low-grade NETs found at the time of retrieval where the risk might be theoretically acceptable (e.g. small carcinoid tumours or multiple small NETs of the stomach), if supported by a good histological evaluation and if the recipient faces an imminent threat to life. In practical terms, however, it is unlikely that a full histological evaluation including Ki-67 and mitotic count could be performed within the donation window to permit accurate grading and prognostication. Without the additional reassurance of documented, long-term, recurrence free survival, any neuroendocrine neoplasm detected at retrieval poses an unacceptable risk to organ transplantation.278 Begum R, Harnois D, Satyanarayana R et al. Retransplantation for donor-derived neuroendocrine tumor. Liver Transplantation, 2010; 17(1): 83-87. 279 Baehner R, Magrane G, Balassanian R et al. Donor origin of neuroendocrine carcinoma in 2 transplant patients determined by molecular cytogenetics. Human Pathol, 2000; 31(11):1425-1429. 280 Göbel H, Gloy J, Neumann J et al. Donor-derived small cell lung carcinoma in a transplanted kidney. Transplantation, 2007; 84(6): 800-802. ×224 Chapter 9: Risk of Transmission of neoplastic diseases. Guide to the Quality and Safety of Organs for Transplantation (7th ed.). European Directorate for the Quality of Medicines & Health Care, Council of Europe, 2018. ×
Donors with a history of Grade 1 or 2 NET, successfully treated with a recurrence-free survival of more than 5 years and without lymph node involvement or metastases, may be considered low risk for malignancy transmission, provided the history is well documented and the person had been closely followed up. Limited evidence exists, however, to guide practice with respect to well-differentiated, low-grade neuroendocrine tumours. Even low-grade NET can metastasise, depending on the location, with late metastases up to 20 years after initial resection possible.281 Factors correlated with higher risk of NET spread include male gender, extra-adrenal location, greater tumour weight, confluent tumour necrosis, vascular invasion and extensive local invasion.282 All information in the donor history should be taken into account and a careful risk-benefit assessment made when determining whether to proceed with donation.281 Szalat A, Fraenkel M, Doviner J et al. Malignant pheochromocytoma: predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical centre. Endocrine, 2011; 39(2): 160-6. ×282 Linnoila RI, Keiser HR, Steinberg SM et al. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol, 1990; 21(11):1168-80. ×
A history of Grade 3 NET poses an unacceptable risk for organ donation regardless of the duration of recurrence- free follow-up, given the known risk of late metastases. Similarly, a history of MiNEN is an unacceptable risk for organ transplantation.
Table 2.11: Classification and grading criteria for neuroendocrine neoplasms of the gastrointestinal tract and hepatopancreatobiliary organs (source: The 2019 WHO classification of tumours of the digestive system, available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7003895/)
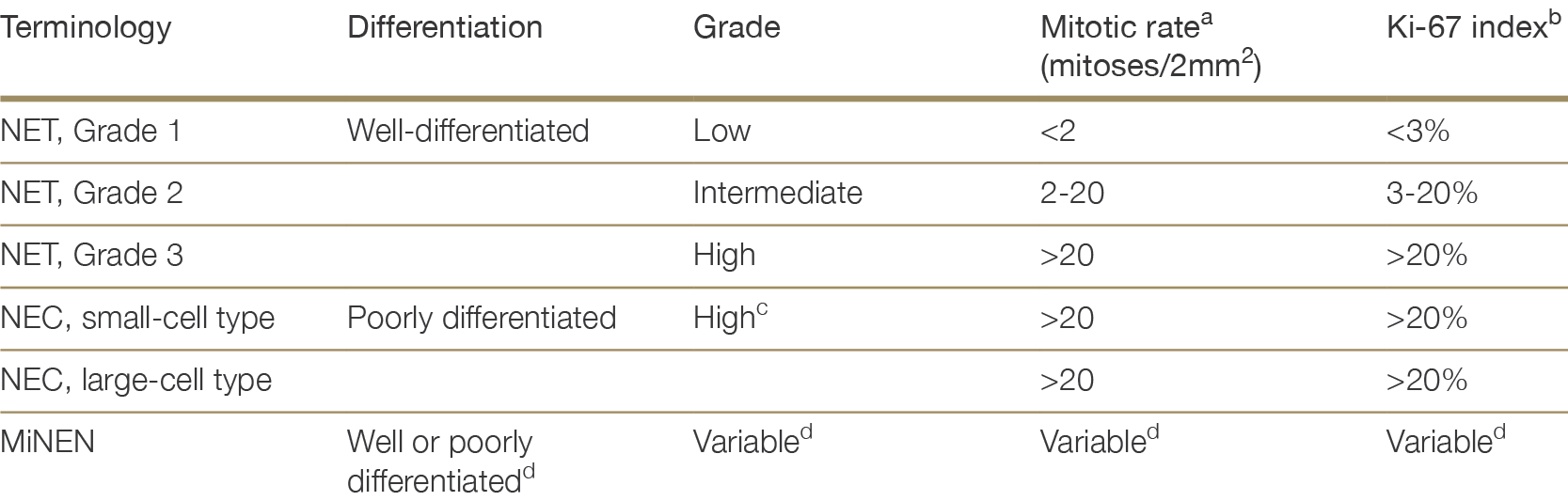
MiNEN, mixed neuroendocrine-non-neuroendocrine neoplasm; NET, neuroendocrine tumour; NEC, neuroendocrine carcinoma
a Mitotic rates are to be expressed as the number of mitoses/2mm2 as determined by counting in 50 fields of 0.2 mm2 (i.e. in a total area of 10 mm2).a No reference text available.×
b The Ki-67 proliferation index value is determined by counting at least 500 cells in the regions of highest labelling (hot-spots), which are identified at scanning magnification; the final grade is based on whichever of the two proliferation indexes places the neoplasm in the higher-grade category.b No reference text available.×
c Poorly differentiated NECs are not formally graded, but are considered high grade by definition.c No reference text available.×
d In most MiNENs, both the neuroendocrine and non-neuroendocrine components are poorly differentiated, and the neuroendocrine component had proliferation indices in the same range as other NECs, but this conceptual category allows for the possibility that one or both components may be well-differentiated; when feasible, each component should therefore be graded separately.d No reference text available.×
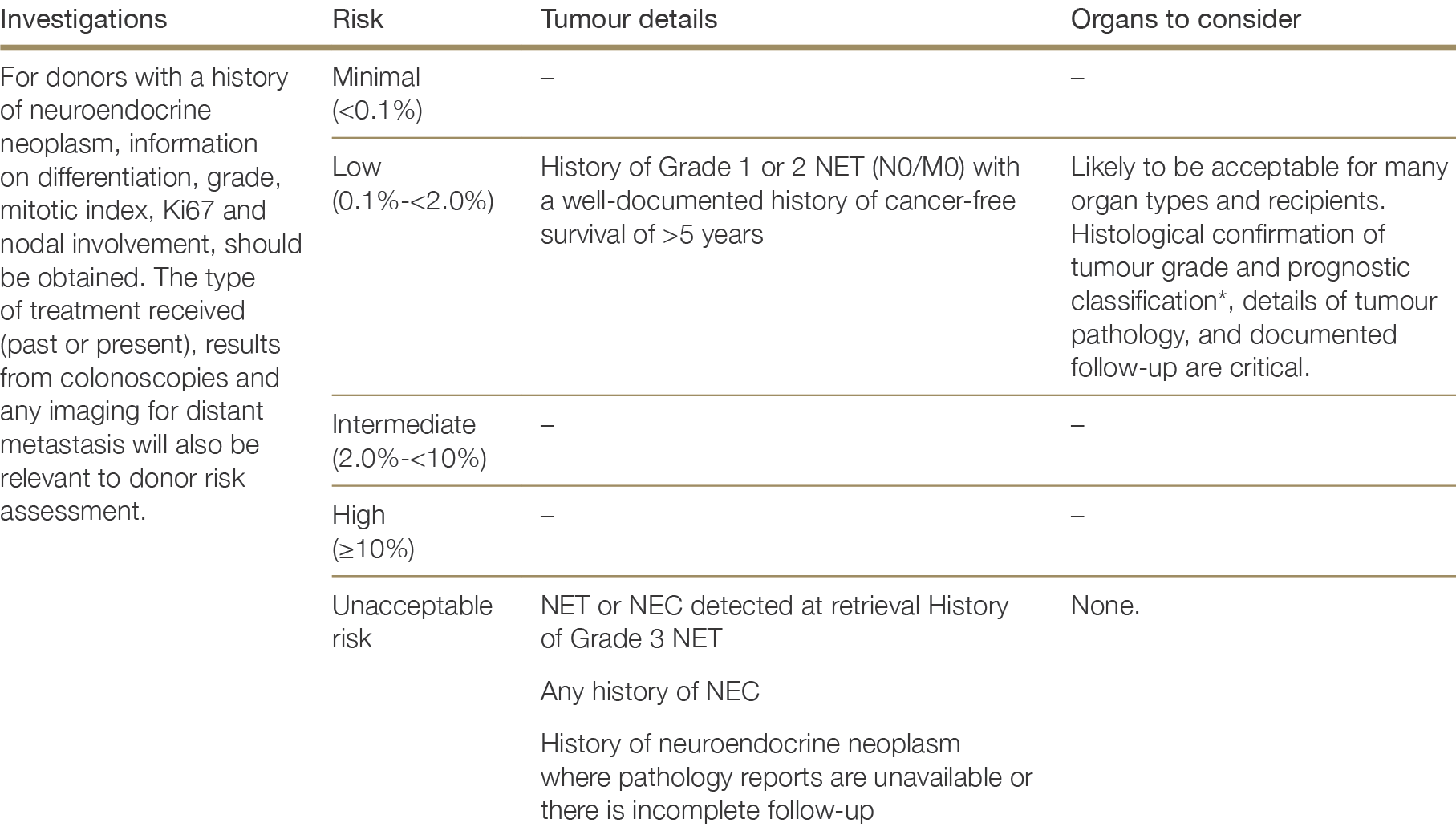
NET, neuroendocrine tumour; NEC, neuroendocrine carcinoma
* WHO 2019 Classification: Grade 1-3 well-differentiated NET defined by histological grade (low/intermediate/high), mitotic rate (<2/2-20/>20mitoses/2mm2) and ki67 index (<3%/3-20%/>20%).
2.4.6.7 Oesophageal, gastric, pancreatic, liver and biliary cancers
All oesophageal, gastric, pancreatic, liver or biliary cancers diagnosed during organ retrievalpose an unacceptable risk to organ transplantation. Given the number of fairly common benign liver tumours, however, it is important to confirm whether a tumour identified at retrieval is malignant before ruling out donation.
If identified in the donor history, treated tumours of these sites are also generally considered an unacceptable risk, given the aggressive nature of these cancers and a high risk of recurrence. Theoretically, risk may decrease following curative therapy and >5 years recurrence-free survival; however, given the high mortality rate for these cancers, this is not a common scenario and only a few isolated case reports of malignancy transmission exist involving donors with oesophageal,283 gastric,284 pancreatic,285,286,287 liver285 or biliary cancer.288283 Taioli E, Mattucci DA, Palmierir S et al. A population-based study of cancer incidence in solid organ transplants from donors at various risk of neoplasia.Transplantation 2007;83(1):13-16. ×284 Fujiwara T, Sakuma Y, Hosoya Y et al. Liver transplantation from a living donor with early gastric cancer. Am J Transplant 2005;5(3):627-9. ×285 Ison MG and MA Nalesnik. An update on donor-derived disease transmission in organ transplantation. Am J Transplant 2011;11(6):1123-30. 286 Kauffman HM, McBride MA, Cherikh WS et al. Transplant tumor registry: donor related malignancies. Transplantation 2002;74(3):358-62. 287 Gerstenkorn C and O Thomusch. Transmission of a pancreatic adenocarcinoma to a renal transplant recipient. Clin Transplant 2003;17(5):473-6. ×285 Ison MG and MA Nalesnik. An update on donor-derived disease transmission in organ transplantation. Am J Transplant 2011;11(6):1123-30. ×288 Georgieva LA, Gielis EM, Hellemans R et al. Single-center case series of donor-related malignancies: rare cases with tremendous impact. Transplant Proc 2016;48(8):2669-2677. ×
In the case of pancreatic cancer, an exception to the recommendations above are intraductal papillary mucinous neoplasms (IPMNs). Intraductal Papillary Mucinous Neoplasms (IPMNs) are considered potential precursors to the development of pancreatic cancer. They are a frequent finding on imaging in the ageing population and are for the most part benign. Persons with branch duct IPMN <3cm without any other “worrisome features”289 of cancer are at low risk and may be considered for donation (excluding pancreas or islets). Branch duct IPMN ≥3cm is thought to carry greater risk of malignancy and whether to proceed will be at the discretion of the teams involved. Donation would usually not be appropriate in the context of features such as a solid component, suspicious nodal disease, or a main duct IPMN >10mm in diameter.289 Tanaka M, Fernández-del Castillo C, Kamisawa T et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology, 2017; 17(5); 738-753. ×
2.4.6.8 Oropharyngeal cancer
Oropharyngeal cancer diagnosed during orgretrievalposes an unacceptable risk to organ transplantation.
For donors with a history of treated oropharyngeal cancer and recurrence free survival >5 years, there may be certain acceptable risk scenarios, although minimal evidence exists on the outcomes of transplantation from donors with a history of oropharyngeal cancer.290 In the context of early non-metastatic lesions that verge on being in situ-type lesions, donation may be acceptable in exceptional circumstances according to recipient need and clinical judgement. Oropharyngeal cancer associated with human papilloma virus (HPV) may also pose a lower risk of donor derived malignancy transmission, given that HPV-positive head and neck cancers have been demonstrated to respond better to treatment,291 have better survival outcomes,292,293 and lower rates of distant metastases than HPV-negative cancers.294,295 Despite a more favourable prognosis, however, recurrence rates for HPV-positive cancers remain at 13-25% within 2 years, and up to 36% within 8 years of treatment.292,296,297290 Kauffman HM, Cherikh WS, McBride MA et al. Deceased donors with a past history of malignancy: an Organ Procurement and Transplantation Network/United Network for Organ Sharing update. Transplantation2007;84(2):272-4. ×291 Spence T, Bruce J, Yip K, Liu FF. HPV associated head and neck cancer. Cancers, 2016; 8(8): 75. ×292 O’Rorke MA, Ellison MV, Murray LJ, et al. Human pa¬pillomavirus related head and neck cancer survival: a sys¬tematic review and meta-analysis. Oral Oncol 2012; 48: 1191–201. 293 Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. JNCI Journal of the National Cancer Institute 2008;100:261–9. ×294 O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human Papillomavirus–Related oropharyngeal cancer according to minimal risk of distant metastasis. JCO 2013;31:543–50. 295 Stenmark MH, Shumway D, Guo C, et al. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope 2017;127:2270–8. ×292 O’Rorke MA, Ellison MV, Murray LJ, et al. Human pa¬pillomavirus related head and neck cancer survival: a sys¬tematic review and meta-analysis. Oral Oncol 2012; 48: 1191–201. 296 Fung N, Faraji F, Kang H, Fakhry C. The role of human papillomavirus on the prognosis and treatment of oropharyngeal carcinoma. Cancer and Metastasis Reviews, 2017; 36:449-461. 297 Nichols A.C., Dhaliwal S.S., Palma D.A. Does HPV type affect outcome in oropharyngeal cancer? J Otolaryngol Head Neck Surg. 2013;42:9. ×
2.4.6.9 Ovarian cancer
Ovarian cancer is considered an unacceptable risk for organ donation.
Although risk may theoretically be diminished in the circumstance of curative surgery and lengthy recurrence-free survival (>10 years), this is an uncommon donation scenario and there are no data to support safe use of such donors.
In the event that an abnormal mass if found on the ovary during retrieval, a diagnosis on frozen section should be sought to inform if organs need to be respectfully disposed.
2.4.6.10 Prostate cancer
In Australia and New Zealand, there is a high prevalence of low-grade, non-aggressive prostate cancer among men over 50 years of age.298 Prostate cancer confined to the prostate has a minimal-to-low risk of transmission and is likely to be present in many male donors without consequence for recipients. An autopsy series of “healthy” organ donors found that 23% of those aged 50-59 years, 35% of those aged 60-69 years, and 46% of those 70-81 years had undiagnosed prostate cancer.299 There is no evidence, however, of increased rates of prostate cancer among transplant recipients relative to the general male population.300,301 From 120 reports of organ transplants from donors with confirmed prostate cancer, there has been only one case of disease transmission.302 In this case, prostate adenocarcinoma was transmitted by heart transplantation from a donor subsequently found to have metastatic disease involving the lymph nodes and adrenal gland.303 A meta-analysis of the outcomes of kidney transplantation from donors with prostate cancer supports the conclusion that the risk of transmitting prostate cancer is lower than the risk of remaining on the waiting list.304298 Cancer in Australia, 2019. Australian Institute of Health and Welfare, 21 Mar 2019 (Cat. No: CAN 123) (available at: https://www. aihw.gov.au/reports/cancer/cancer-in-australia-2019/data). ×299 Yin M, Bastacky S, Chandran U, et al. Prevalence of incidental prostate cancer in the general population: A study of healthy organ donors. J Urol, 2008;179:892–895. ×300 Rosales B, De La Mata N, Vajdic C et al. Cancer mortality in kidney transplant recipients: An Australian and New Zealand population-based cohort study, 1980-2013. International Journal of Cancer, 2020; 146:2703-2711. 301 Na R, Grulich AE, Meagher NS et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. American Journal of Transplantation, 2013; 13:174-183. ×302 Doerfler A, Tillou X, Le Gal S, Desmonts A, Orczyk C, Bensadoun H. Prostate cancer in deceased organ donors: a review. Transplant Rev, 2014; 28(1): 1-5. ×303 Loh E Couch FJ, Hendricksen C et al. Development of donor-derived prostate cancer in a recipient following orthotopic heart transplantation. JAMA, 1997; 277(2):133-7. ×304 Dholakia S, Johns R, Muirhead L, Papalois V, Crane J. Renal donors with prostate cancer, no longer a reason to decline. Transplant Rev, 2016; 30(1): 48-50. ×
For donors with no history of prostate cancer, routine prostate specific antigen (PSA) screening is not recommended. PSA results in this group are likely to be unreliable due to elevation caused by catherization and routine PSA testing would likely result in unnecessary investigations. In addition, numerous benign prostate pathologies will cause an elevation in PSA.305305 Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Adult Urology, 1999; 53(3): P581-589. ×
For donors with a known history of prostate cancer, PSA testing may be appropriate in situations where it is necessary to rule out prostate cancer recurrence or spread; in this circumstance, a PSA level that is undetectable or below 0.1 usually indicates minimal risk of transmission. If PSA is elevated (>6.5), organs may still be acceptable for many recipients. Isolated PSA results must, however, be interpreted with caution. In donors with a history of prostate cancer, results should ideally be interpreted in the context of past measures of PSA for that individual. Of greater importance to risk assessment is the prostate cancer grade, stage and disease-free interval.
For donors diagnosed with prostate cancer at the time of retrieval, or where there is a report of prostate cancer in the donor history, whether to proceed with donation will depend on the grade (Gleason score), stage and cancer-free survival for those who have previous surgical treatment. Grade group 1 (Gleason score 6) prostate cancer – which is the majority of prostate cancer diagnosed in Australia and New Zealand – is a largely indolent disease and is associated with minimal risk of transmission by organ donation.295 There is an almost-zero risk of transmission of prostate cancer out of the prostate to a secondary site when Gleason score is 6.306 Where Grade group 1 prostate cancer is detected at the time of retrieval or in the donor history, all organs may be safely transplanted. A donor history of treated Grade group 2/3 (Gleason score 7) prostate cancer may also be considered minimal risk, provided the tumour was organ-confined and the donor has been cancer-free for more than 3 years.295 Stenmark MH, Shumway D, Guo C, et al. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope 2017;127:2270–8. ×306 Ross HM, Kryvenko ON, Cowan JE et al. Do adenocarcinomas of the prostate with Gleason score (GS) <6 have the potential to metastasize to lymph nodes? Am J Surg Pathol, 2012; 36(9):1346-52. ×
Where the donor history indicates the donor has previously received anti-androgen therapy (often delivered in conjunction with radiation therapy), consult with a urologist/oncologist before proceeding to donation. Anti-androgen therapy as a single agent therapy is suggestive of more aggressive disease prior to treatment, therefore risk of transmission is higher, even if PSA is low-to-undetectable at the time of donor evaluation.
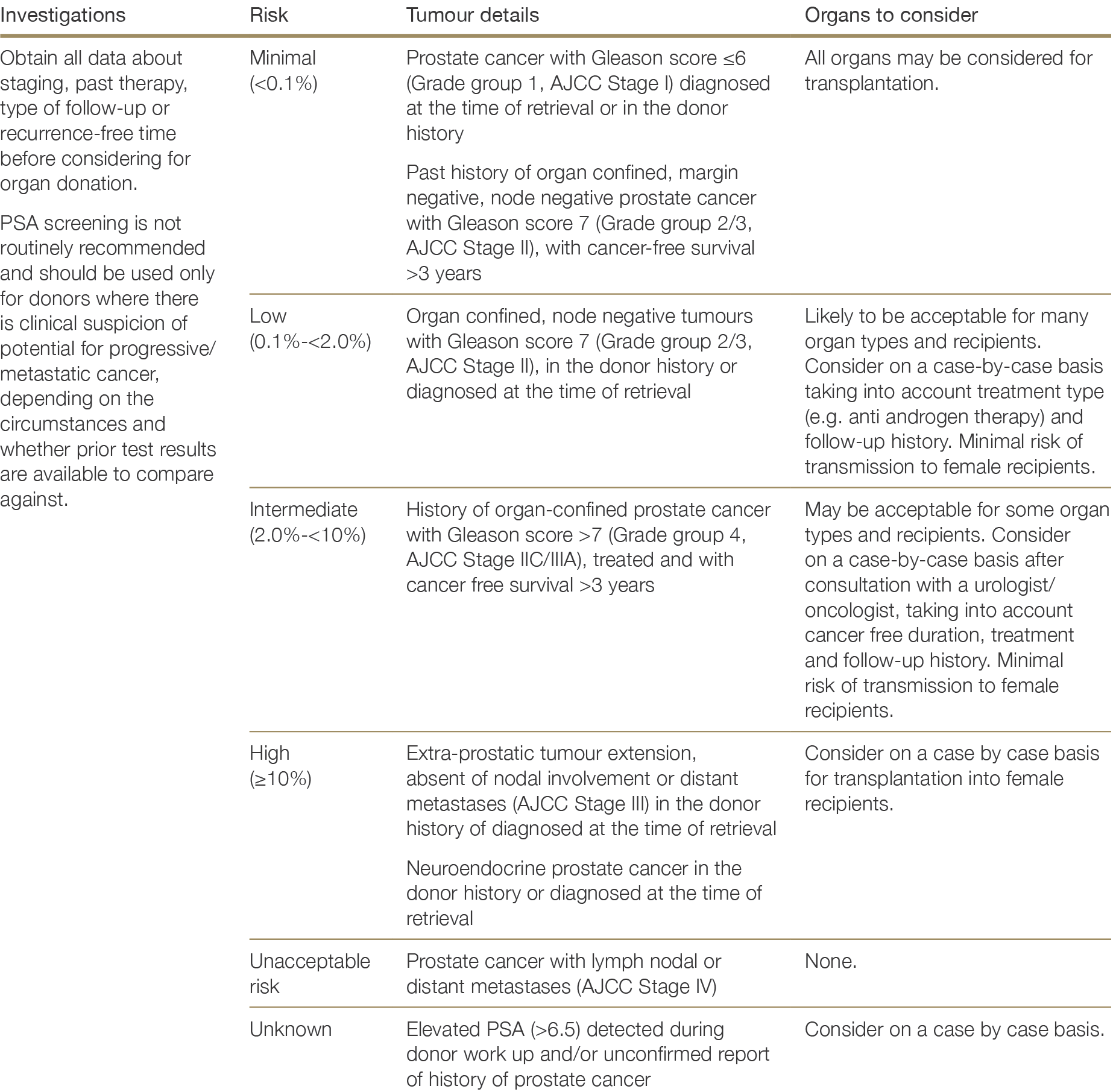
2.4.6.11 Renal cell carcinoma
Suspicious lesions detected during retrieval
If a solid and/or suspicious lesion is found on the kidney at the time of organ retrieval or bench-top assessment, other organs should be assessed for possible involvement and other organ team members notified. Frozen section or urgent histopathology with limited immunostaining may be available to guide decisions. In a single-centre Italian study by Ambrosi et al, 51 incidental solid renal lesions (0.3-3.2cm) identified at the time of retrieval were assessed with frozen section.307 Of these, 26 (51%) were found to be neoplastic and 25 (49%) were benign. Of all the renal tumours assessed with formal histopathology in this study, 69% were malignant and 31% benign (e.g. angiomyelolipoma, oncocytoma).307 F. Ambrosi, C. Ricci, D. Malvi, et al., “Pathological Features and Outcomes of Incidental Renal Cell Carcinoma in Candidate Solid Organ Donors,” Kidney Research and Clinical Practice 39, no. 4 (2020): 487–494, doi:10.23876/j.krcp.20.050 ×
In certain situations, especially where cold ischemic time is prolonged, urgent frozen section/histopathological review may not be available or impractical. In these situations, non-renal organs and the contralateral kidney may be considered on a case-by-case basis after consulting with a subject expert and provided a recent imaging shows no evidence of involvement of these organs. Ideally, informed consent of the recipient should be sought prior to proceeding.
Renal cell carcinoma detected during retrieval
There are several case series documenting the safe transplantation of kidneys following renal cell carcinoma (RCC) resection for tumours up to 4cm in size detected at the time of organ retrieval. Pavlakis et al report no cases of malignancy transmission from 21 kidneys following excision of tumour (0.1–2.1cm), as well as no cases of transmission from the transplantation of 47 contralateral kidneys and 198 non-renal organs.307 Yu et al report 97 cases of kidney transplantation after RCC resection of tumours up to 4cm without transmission. 22 cases of normal contralateral kidney transplants were reported, with one case of transmission (although the diagnosis of the original donor cancer has been questioned in this instance).308 In the latest systematic review by Villani et al encompassing most available case reports and series, the risk of malignant recurrence after tumour resection prior to transplantation was 2.7%, where utilised kidneys included grade I-II (81.6%) and small tumours (1.26cm) with a mean follow up of 39.9 months.309307 F. Ambrosi, C. Ricci, D. Malvi, et al., “Pathological Features and Outcomes of Incidental Renal Cell Carcinoma in Candidate Solid Organ Donors,” Kidney Research and Clinical Practice 39, no. 4 (2020): 487–494, doi:10.23876/j.krcp.20.050 ×308 Yu N, Fu S, Fu Z et al. Allotransplantating donor kidneys after resection of a small renal cancer or contralateral healthy kidneys from cadaveric donors with unilateral renal cancer: a systematic review. Clinical Transplantation, 2014; 28(8):8-15. ×309 Villani, V., Kulkarni, R. D., Fair, J. H., & Shanmugarajah, K. (2024). Kidney Transplantation From Donors With Malignant Renal Masses: A Systematic Review. Clinical transplantation, 38(11), e70020. https://doi.org/10.1111/ctr.70020 ×
In the case of a solitary, well-differentiated RCC, the risk of malignancy transmission is minimal (<0.1%) where the tumour is ≤1.0 cm in size, or low (approximately 0.1 – 2.0%) for tumours >1.0 cm to ≤4 cm in size. All organs may be considered for transplantation in the context of an T1a RCC ≤4 cm with nucleolar grade I-II, including transplantation of the affected kidney following tumour resection, provided satisfactory margins are achieved and imaging of the urinary tract has excluded multifocal cancer deposits.202,310,311 When imaging is not available, the risk assessment may include a discussion with a subject expert.202 Transplantation of Organs from Deceased Donors with Cancer or a History of Cancer. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2014. 310 Nicol DL, Preston JM, Wall DR, et al. Kidneys from patients with small renal tumours: a novel source of kidneys for transplantation. BJU Int. 2008;102(2):188-92. 311 Nalesnik MA, Woodle ES, Dimaio JM, et al. Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant, 2011;11(6):1140-7. ×
There are some caveats to utilising these kidneys. Sprott et al. reported a return-to-theatre risk at as high as 39% due to bleeding or urine leak after resection.312 Although the risk is lower in other reports, this should be a part of the discussion with the potential recipient. Furthermore, careful and formal histopathological analysis with immunohistochemistry is required, as a small percentage of incidental tumours will have unacceptable features such as collecting duct, sarcoma, sarcomatoid or rhabdoid differentiation, or molecularly defined characteristics. Patients with adverse findings on the final histopathology may require early graft nephrectomy. Frozen section and formal histopathology results should be made available to other organ teams even though the risk of metastasis for a small renal cell carcinoma is very small.313,314,315,316,317,318,319,320,321312 Sprott, P., Hibberd, A. D., Heer, M. K., Trevillian, P. R., Clark, D. A., Johnson, D. W., Oldmeadow, C., Chiu, S., & Attia, J. R. (2019). Assessment of Restored Kidney Transplantation Including the Use of Wider Criteria for Accepting Renal Donors After Cancer Excision. Transplantation direct, 5(11), e498. https://doi.org/10.1097/TXD.0000000000000946 ×313 Meyding-Lamade U, Krieger D, Schnabel P et al. Cerebral metastases of an allogenic renal cell carcinoma in a heart recipient without renal cell carcinoma. J Neurol 1996;243(5):425-7. 314 Sack FU, Lange R, Mehmanesh H et al. Transferral of extrathoracic donor neoplasm by the cardiac allograft. J Heart Lung Transplant 1997;16(3):298-301. 315 Barrou B, Bitker MO, Delcourt A et al. Fate of a renal tubulopapillary adenoma transmitted by an organ donor. Transplantation 2001;72(3):540-41. 316 Moench K, Breidenbach T, Fischer-Fröhlich C-L et al. 6-year survey of organ donors with malignancies in Germany. Transplantation 2012;94(10S):527 317 Pavlakis M, Michaels MG, Tlusty S et al. Renal cell carcinoma suspected at time of organ donation 2008-2016: a report of the OPTN ad hoc Disease Transmission Advisory Committee Registry. Clin Transplant 2019;33(7):e13597. 318 Serralta AS, Orbis FC, Sanjuan FR et al. If the donor had an early-stage genitourinary carcinoma and the liver has already been implanted, should we perform the transplantectomy? Liver Transplant 2003;9(12): 1281-5. 319 Moench K, Breidenbach T, Fischer-Fröhlich C-L et al. 6-year survey of organ donors with malignancies in Germany. Transplantation 2012;94(10S):527 320 Pavlakis M, Michaels MG, Tlusty S et al. Renal cell carcinoma suspected at time of organ donation 2008-2016: a report of the OPTN ad hoc Disease Transmission Advisory Committee Registry. Clin Transplant 2019;33(7):e13597. 321 Serralta AS, Orbis FC, Sanjuan FR et al. If the donor had an early-stage genitourinary carcinoma and the liver has already been implanted, should we perform the transplantectomy? Liver Transplant 2003;9(12): 1281-5. ×
Ongoing surveillance by annual ultrasound is recommended for (i) recipients of kidneys with a resected tumour, given the small risk of recurrence and/or new primary cancer and (ii) recipients of contralateral kidneys, given the risk of developing a new primary.
Bosniak Cysts detected during retrieval
Bosniak cysts can only be defined by radiological imaging with contrast, most typically CT or MRI, which are performed prior to organ procurement.322 The Bosniak classification of renal cysts describes radiological characteristics of a renal cyst, such as the presence of calcification or septum, septum with contrast enhancement or solid component (either enhancing or non-enhancing) of the lesion. In a systematic review by Sevcenco et al, the pooled risk of malignancy was 3.2% for Bosniak cyst I, 6 % for Bosniak cyst II, 6.7 % for Bosniak cyst IIF, 55.1% for Bosniak cyst III and 91 % for Bosniak cyst IV. 323 Lesions categorised as Bosniak I-II should not be manipulated prior to transplantation, whilst Bosniak cyst IIF could be considered for either monitoring or removal prior to transplantation. For lesions <4cm with Bosniak cyst III–IV, tumour resection prior to transplantation should be offered. The malignant potential of the Bosniak cysts is thought to be driven by the solid component within the lesion and therefore, in direct size comparison to solid lesions, Bosniak cysts may have lower risk of malignancy and transmission.322 Silverman, S. G., Pedrosa, I., Ellis, J. H., Hindman, N. M., Schieda, N., Smith, A. D., Remer, E. M., Shinagare, A. B., Curci, N. E., Raman, S. S., Wells, S. A., Kaffenberger, S. D., Wang, Z. J., Chandarana, H., & Davenport, M. S. (2019). Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology, 292(2), 475–488. https://doi.org/10.1148/radiol.2019182646 ×323 Sevcenco, S., Spick, C., Helbich, T. H., Heinz, G., Shariat, S. F., Klingler, H. C., Rauchenwald, M., & Baltzer, P. A. (2017). Malignancy rates and diagnostic performance of the Bosniak classification for the diagnosis of cystic renal lesions in computed tomography - a systematic review and meta-analysis. European radiology, 27(6), 2239–2247. https://doi.org/10.1007/s00330-016-4631-9 ×
Renal cell carcinoma in the donor history
If the donor has been diagnosed with RCC <5 years prior to being considered for organ donation, the same risk thresholds defined above for RCC diagnosed at the time of organ retrieval apply. If the diagnosis in the donor was made >5 years ago and there has been recent follow up and/or CT scan showing nothing of concern, risk of transmission may be lower. Follow-up surveillance of recipients is recommended.
Donors with a history of treated RCC >4cm to 7cm in size with nucleolar grade I-II, with cancer-free survival for >5 years may be considered for transplantation of non-renal organs, provided that a recent CT scan shows nothing of concern. Donation of the contralateral kidney in the context of a history of RCC >4–7cm should be considered on a case-by-case basis, after reviewing donor history and follow-up.
A history of invasive RCC (Stage III or IV) or unconventional disease presents an unacceptable risk to organ transplantation.
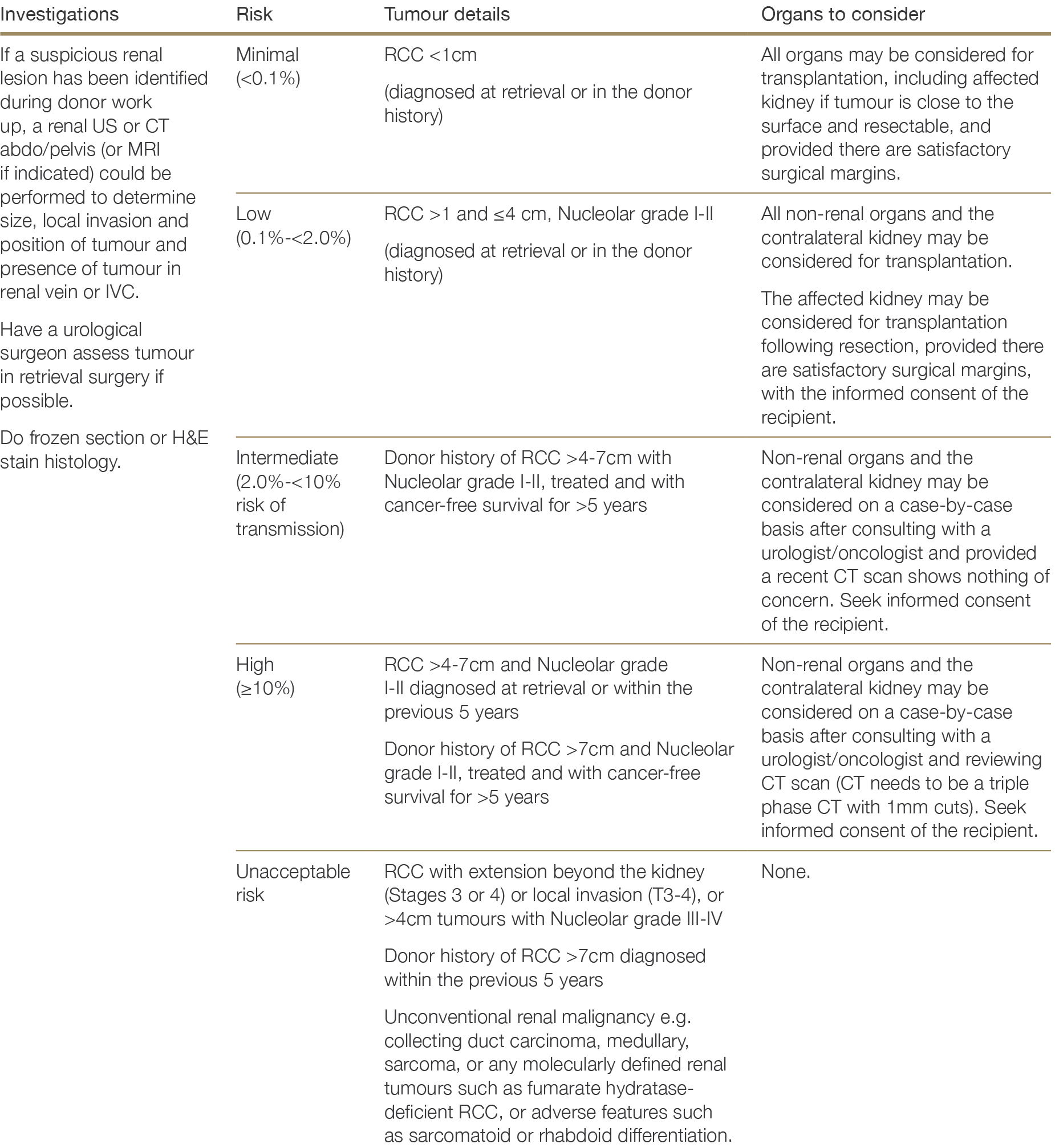
2.4.6.12 Sarcoma
For recommendations regarding Kaposi’s sarcoma (Human Herpes Virus 8) see Section 2.3.2.7.
Sarcoma detected during retrieval
Given the typically aggressive nature of these tumours, sarcoma diagnosed during retrieval poses an unacceptable risk to organ transplantation. While there are a number of low-grade sarcomas, obtaining a definitive diagnosis in the timeframe required for transplantation is unlikely to be feasible.
Sarcoma in the donor history
Isolated case reports from the literature demonstrate fatal outcomes of transplantation in the context of unrecognised donor sarcoma.324,325 However, given the vast array of possible soft tissue and bone sarcomas and their heterogeneity with regards risk of recurrence and potential risk of transmission to recipients, potential donors with a history of treated sarcoma and a substantial recurrence-free period should be considered on a case by case basis.324 Detry O, De Roover A, de Leval et al. Transmission of an undiagnosed sarcoma to recipients of kidney and liver grafts procured in a non-heart beating donor. Liver Transpl, 2005; 11(6):696-9. Thoning J, Liu J, Bistrup C et al. Transmission of angiosarcomas from a common multiorgan donor to four transplant recipients. Am J Transplant, 2013; 13(1):167-73. 325 Thoning J, Liu J, Bistrup C et al. Transmission of angiosarcomas from a common multiorgan donor to four transplant recipients. Am J Transplant, 2013; 13(1):167-73.×
Sarcoma type and associated long-term risk of recurrence, treatment type and era, and length of follow-up are key considerations in assessing transmission risk. As sarcomas are typically very aggressive, a person who is very late into follow-up (20-30 years) is unlikely to carry a high residual risk of cancer transmission.326 Long term recurrence rates have reduced further in the current era due to improved treatment protocols. Organs from a donor with a distant history (30+ years) of Ewing sarcoma have previously been transplanted in Australia with good outcomes. The long-term data on recurrence of different sarcoma types should be taken into account when evaluating potential donors – particularly in the case of childhood cancers such as Ewing sarcoma.326 Wasilewski-Masker K, Liu Q, Yasui Y et al. Late recurrence in pediatric cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst, 2009; 10(24):1709-20. ×
2.4.6.13 Thyroid cancer
Over the past 30 years, the annual incidence of thyroid cancer diagnosed in Australia has steadily increased, from an age-standardised incidence rate of 3.3 per 100,000 in 1990, to an estimated rate of 14 cases per 100,000 in 2020.275 Similar increases in the incidence of thyroid cancer have been reported globally, mostly driven by increases in lower stage papillary thyroid cancer and incidental findings of micro-papillary thyroid cancers during surgery for other conditions of the thyroid.327 In Australia and globally, mortality from thyroid cancer has remained steady, suggesting a degree of overdiagnosis of thyroid cancer in the general population.328275 Cancer data in Australia, web report, Australian Institute of Health and Welfare, 13 Nov 2020 (Cat. No: CAN 122) (available at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary) ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×328 Davies I & Welch HG. Increasing incidence of thyroid cancer in the United States 1973-2002. JAMA, 295; 2164-2167. ×
The main types of thyroid cancer are:
Differentiated (including papillary, follicular and Hürthle cell)
Medullary
Anaplastic.
Papillary thyroid cancer accounts for approximately 80% of thyroid cancers, and follicular thyroid cancer for another 10%.329 These cancers generally grow very slowly and are typically low-stage at diagnosis, with only localised spread.330 Overall, five year relative survival in people with thyroid cancer is 97% and the prognosis for differentiated thyroid cancer is very good until advanced stages of disease.331,332 Distant metastases develop in 5-23% of people with differentiated thyroid cancer; when these do occur, it is mainly in the lungs and bones.327 Current clinical practice guidelines recommend total thyroidectomy plus radioiodine for differentiated thyroid tumours greater than 4cm in diameter or tumours of any size associated with multifocal disease, bilateral disease, extra-thyroidal spread (pT3 and pT4a), familial disease or nodal involvement/distant metastases.327 Post-operative risk of recurrence is low where there is complete resection and the tumour does not have aggressive histology.333 Late recurrences can occur but can be successfully treated.327329 https://www.cancer.org/cancer/thyroid-cancer/about/what-is-thyroid-cancer.html https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/cancer-statistics-nsw#//analysis/incidence/ ×330 https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/cancer-statistics-nsw#//analysis/incidence/×331 https://www.canceraustralia.gov.au/affected-cancer/cancer-types/thyroid-cancer/thyroid-cancer-australia-statistics 332 Verberg FA, Mäder U, Tanase K et al. Life expectancy is reduced in differentiated thyroid cancer patients >45 years with extensive local tumour invasion, lateral lymph node or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metabolism, 2013; 98:172-180. ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×333 Cooper DS, Doherty GM, Haugen BR et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid, 2009; 19: 1167-1214. ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×
The risk of transmission of differentiated thyroid cancer from a donor to a solid organ recipient relates to the size of the tumour and whether it has spread beyond the thyroid. Differentiated thyroid cancers of up to 4cm in size and confined to the thyroid pose minimal risk of donor-derived malignancy transmission, even if detected at the time of organ retrieval. It is important to note that thyroid cancers do not seem to be affected by immunosuppression: pre-existing thyroid cancers do not show increased rates of progression and incidence of thyroid cancer is not elevated in recipients of non-kidney organs – in kidney transplant recipients the incidence of thyroid cancer is not above that of dialysis patients.334,335 In addition, even widely metastatic differentiated thyroid cancer is amenable to curative therapy, depending on its histology.327 Hence, even in the event of donor-derived transmission, there could be a reasonable expectation of curative treatment.334 Van Leeuwen MT, Webster A, McCredie MRE et al. Effect of reduced immunosuppression after kidney transplant on risk of cancer: population based retrospective cohort study. BMJ, 2010; 340: c570. Na R, Grulich AE, Meagher NS et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant, 2013; 13:174-183. 335 Na R, Grulich AE, Meagher NS et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant, 2013; 13:174-183.×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×
Medullary thyroid cancer (MTC) accounts for <5% of thyroid cancer and may be sporadic (approximately 75% of MTC) or familial (approximately 25%).327 Familial MTC has an earlier age of onset and is more aggressive, with a tendency to spread to the lungs, liver or bones. In confirmed cases of MTC, treatment is total thyroidectomy and central compartment node dissection. Lifelong follow-up is required and there is the potential for local recurrence or distant metastases.327 For this reason, a history of MTC poses an intermediate-to-high risk to organ transplantation. A history of MTC with nodal involvement or distant metastasis is an unacceptable risk to organ transplantation.327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×
Anaplastic thyroid cancer is a rare (approximately 2% of all thyroid cancers) and aggressive form of thyroid cancer that primarily occurs in people over 65 years of age.327 All anaplastic thyroid cancers are Stage IV with a poor prognosis (5-year relative survival of 7%).327 Any history of anaplastic thyroid cancer poses a unacceptable risk to organ transplantation.327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×327 Perros P, Colley PP, Boelaert K et al. British thyroid association guidelines for the management of thyroid cancer. Clinical Endocrinology, 2014; 81(Suppl 1) ×
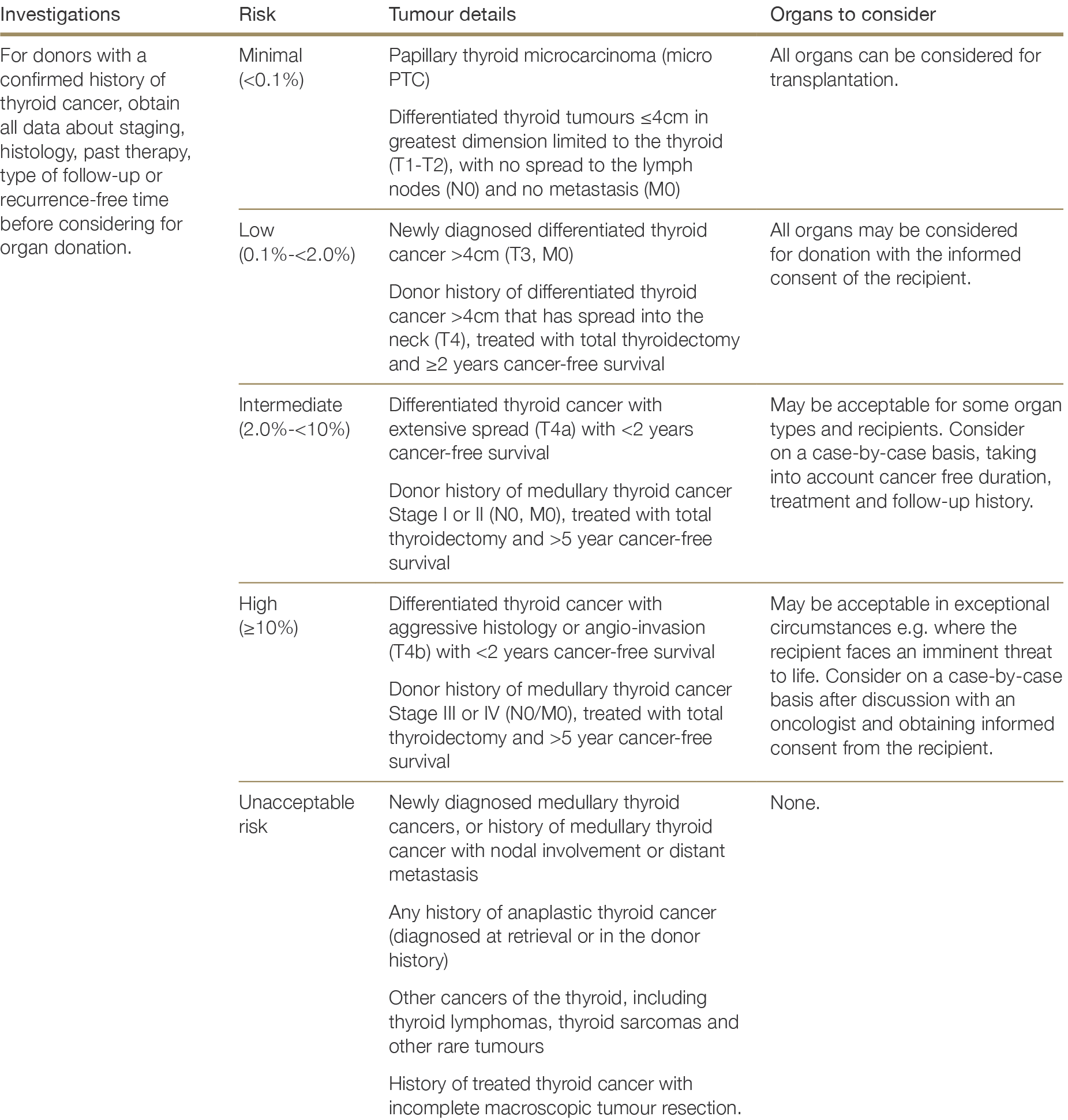
2.4.6.14 Urothelial carcinoma
Superficial, non-invasive papillary carcinoma of the bladder is not a contraindication to organ donation; however, it is important to establish that the cancer is low-grade before proceeding with donation. If urothelial cancer is diagnosed during retrieval, the advice of a urologist should be sought in assessing the tumour prognosis/ behaviour in the donor.
If urothelial carcinoma is present in the donor history, donors should only be considered if there has been strict follow-up after primary diagnosis and complete histological information is available, given the high risk of recurrence of these tumours. Risk of recurrence increases (i) with increasing Stage and Grade, (ii) with greater tumour diameter, (ii) where multiple tumours are present, (iv) where there have been one or more prior recurrences, and/or (v) where there is concurrent carcinoma in situ. 336 A history of muscle-invasive bladder cancer (T2 and above) poses an unacceptable risk to organ transplantation, unless there is documented evidence of prolonged cancer-free survival. It is possible that the risk of transmission is lower from donors who have previously had a cystectomy and/or chemoradiation and have been cancer-free for >5 years, after which point the risk of recurrence is very low for patients with T1 or T2 disease.337,338,339 For patients with T3 or T4 disease, the risk of recurrence at 5–10 years post-cystectomy is higher, at around 5%.339 However, no data are available on the outcomes of transplantation in such circumstances.336 Babjuk M, Burger M, Compérat EM et al. European Association of Urology Guidelines and non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 update. European Urology, 2019; 76: 639-657. ×337 May M, Helke C, Nitzke T et al. Survival rates after radical cystectomy according to tumour stage of bladder carcinoma at first presentation. Urol Int, 2004; 72:103-111. 338 de Vries RR, Nieuwenhuijzen JA, Vincent A et al. Survival after cystectomy for invasive bladder cancer. Eur J Surg Oncol, 2010; 36(3):292-297. 339 Madersbacher S, Hochreiter W, Burkard F et al. Radical cystectomy for bladder cancer today – a homogenous series without neoadjuvant therapy. J Clin Oncol, 2003; 21 (4): 690-696. Möricke A, Zimmermann M, Reiter A et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia, 2010; 24:265-284. ×339 Madersbacher S, Hochreiter W, Burkard F et al. Radical cystectomy for bladder cancer today – a homogenous series without neoadjuvant therapy. J Clin Oncol, 2003; 21 (4): 690-696. Möricke A, Zimmermann M, Reiter A et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia, 2010; 24:265-284. ×
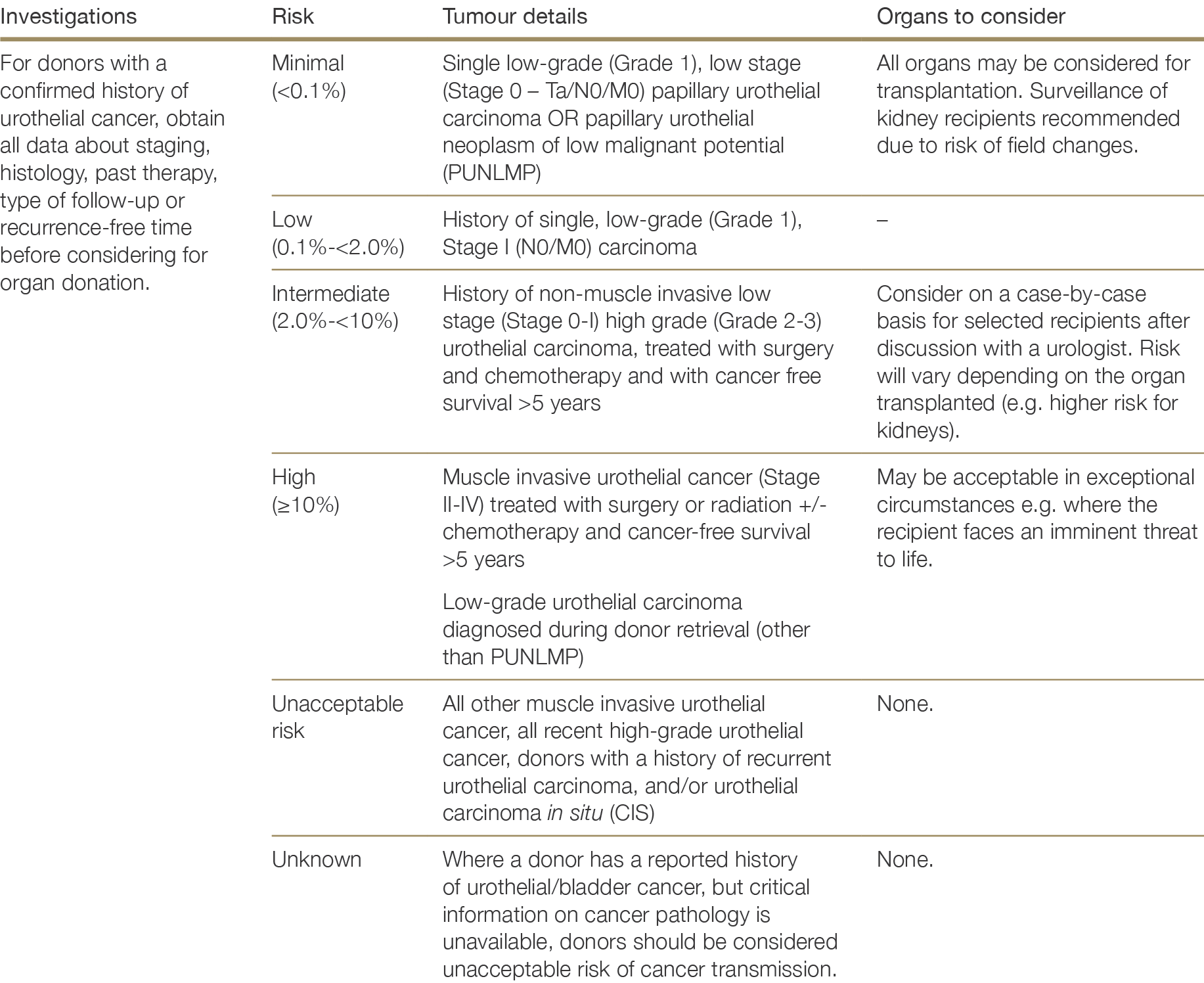
2.4.6.15 Uterus and uterine cervix cancer
Invasive uterus or uterine cervix cancer (Stage I or higher) diagnosed at the time of organ retrieval poses an unacceptable risk to organ transplantation. For donors with a history of invasive uterus or uterine cervix cancers, transmission risk may diminish after a disease-free interval of >5 years, however there are minimal data from the literature to support a recommendation, and donors should be assessed on a case-by-case basis.
Pre-cancerous cervical cell changes pose little to no risk to organ transplantation. Low grade squamous intraepithelial lesions (previously graded as CIN1) usually disappear without treatment. High grade squamous intraepithelial lesions (previously graded as CIN2 or 3) and adenocarcinoma in situ are pre-cancerous changes but pose minimal risk of donor-derived malignancy transmission.
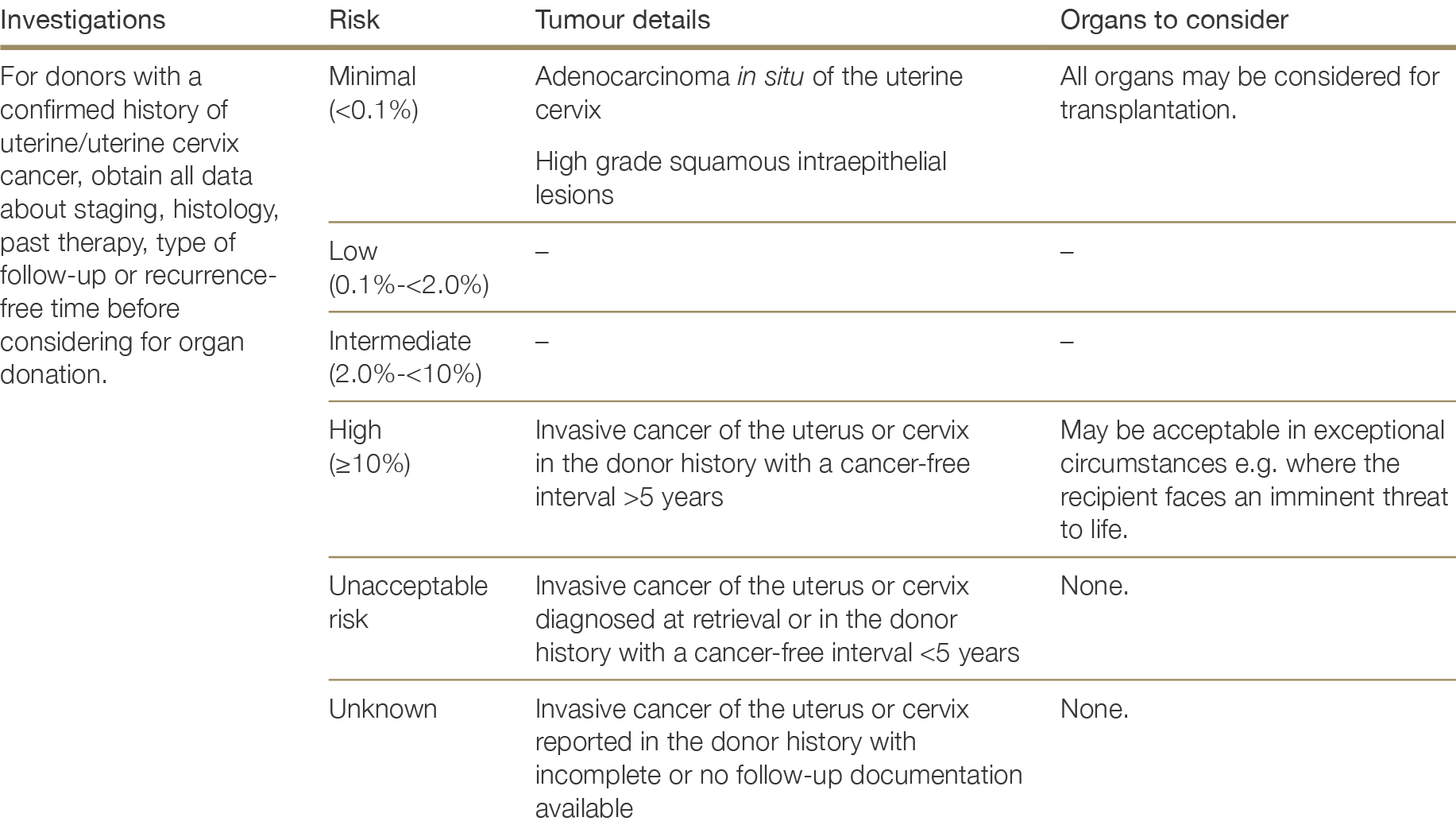
2.4.7 Haematological cancers
Current haematological malignancy poses an unacceptable risk to organ transplantation.
A recent donor history of treated leukaemia, lymphoma or plasmacytoma and <5 years recurrence-free survival is also an unacceptable risk to donation.340 In the context of donors with a history of treated acute leukaemia or lymphoma and 5 or more years of recurrence-free survival, including childhood leukaemia and Hodgkin’s lymphoma survivors, seek expert advice from a haematologist on likely transmission risk.340 Möricke A, Zimmermann M, Reiter A et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia, 2010; 24:265-284.×
Utilisation of donors with a history of low-grade haematological malignancies and other clonal haematological disorders has been proposed for certain recipients.341 These include monoclonal gammopathy of uncertain significance (MGUS) and myeloproliferative neoplasms (MPN) – including polycythaemia vera, essential thrombocythaemia and monoclonal B cell lymphocytosis – all of which have a long natural median survival342,343 and are becoming more common among donors as the donor population ages. MGUS are found in approximately 4% of the population older than 50 and are usually benign and asymptomatic,344 hence are likely to be present in many organ donors without consequence for recipients. There have, however been reported instances of donor-derived lymphoproliferative disorders in recipients of solid organ transplants from donors with MGUS,345 hence the risk associated with such donors is not negligible. Donors with a known history of MGUS may be acceptable in certain circumstances, following advice from a haematologist. There are no data on transmission risk and the outcomes of transplantation from donors with MPN. Given that the goal of MPN treatment is usually symptom control rather than cure, even in treated patients there remains the potential for clonogenic stem cells to be transmitted with donor organs. It is not known how a transmitted MPN would behave in the immunosuppressed recipient, hence transplantation of organs from donors with a history of MPN is not recommended.341 Chapter 9: Risk of Transmission of Neoplastic Diseases. Guide to the Quality and Safety of Organs for Transplantation (7th ed.). European Directorate for the Quality of Medicines & Health Care, Council of Europe, 2018. ×342 Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th ed.). World Health Organisation Press, Geneva, 2008. 343 Rawstron AC, Bennett FL, O’Connor SJ et al. Monoclonal B-Cell Lymphocytosis and Chronic Lymphocytic Leukemia. N Engl J Med 2008;359(6): 575-583. ×344 Dispenzieri A, Katzmann JA, Kyle RA et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet, 2010; 375:1721-1728. Felldin M, Ekberg J, Polanska-Tamborek D et al. Donor monoclonal gammopathy may cause lymphoproliferative disorders in solid organ transplant recipients. Am J Transplant, 2016; 16: 2676-2683. ×345 Felldin M, Ekberg J, Polanska-Tamborek D et al. Donor monoclonal gammopathy may cause lymphoproliferative disorders in solid organ transplant recipients. Am J Transplant, 2016; 16: 2676-2683.×
2.4.8 Suspicion of malignancy transmission in an organ recipient
2.4.8.1 Actions in the case of suspected or confirmed donor-transmitted malignancy
The potential for donor-derived malignancy may be identified at any one of three points on the donation pathway:
Before organ retrieval (during donor assessment and workup)
At organ retrieval (e.g. neoplasia discovered during retrieval surgery)
After the transplantation of at least one organ.
When malignancy is detected in the donor during workup, surgery, pretransplant organ preparation, or in the immediate post-transplant period, this is an urgent issue requiring immediate notification of all affected transplant units, DonateLife and eye and tissue banks.
In cases where organs have already been transplanted and histology reveals a malignancy (e.g. incidental cancer in a lung lobe discarded due to size reduction), a full donor autopsy should be requested whenever possible to obtain detailed information about tumour origin and dissemination. This will not be necessary in cases of small primary renal cell carcinoma found in one kidney, which would not preclude the transplantation of other organs.
Early diagnosis of donor-transmitted malignancy (within 6 weeks of transplantation) is associated with better survival outcomes. Analysis of the UK transplant registry found that 20% of recipients with a donor-derived cancer of the transplanted organ died as a direct result of cancer; however, where the donor transmitted cancer was detected early, there were no deaths related to the donor transmitted cancer.202202 Transplantation of Organs from Deceased Donors with Cancer or a History of Cancer. Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), UK Government Department of Health, London, UK, 2014. ×
Where the possibility of a donor-derived cancer is identified in a recipient at some time after transplantation, DonateLife and Organ Donation New Zealand need to be informed as soon as possible and the origin of the cancer investigated.
Optimal recipient management in the event of a donor transmitted malignancy will depend on the type of tumour, the organ transplanted, the time from transplantation to diagnosis and the patient’s immunosuppression regimen. How to proceed will be a joint decision of physician and recipient.
Table 2.11: Actions in the event of confirmed diagnosis of donor malignancy
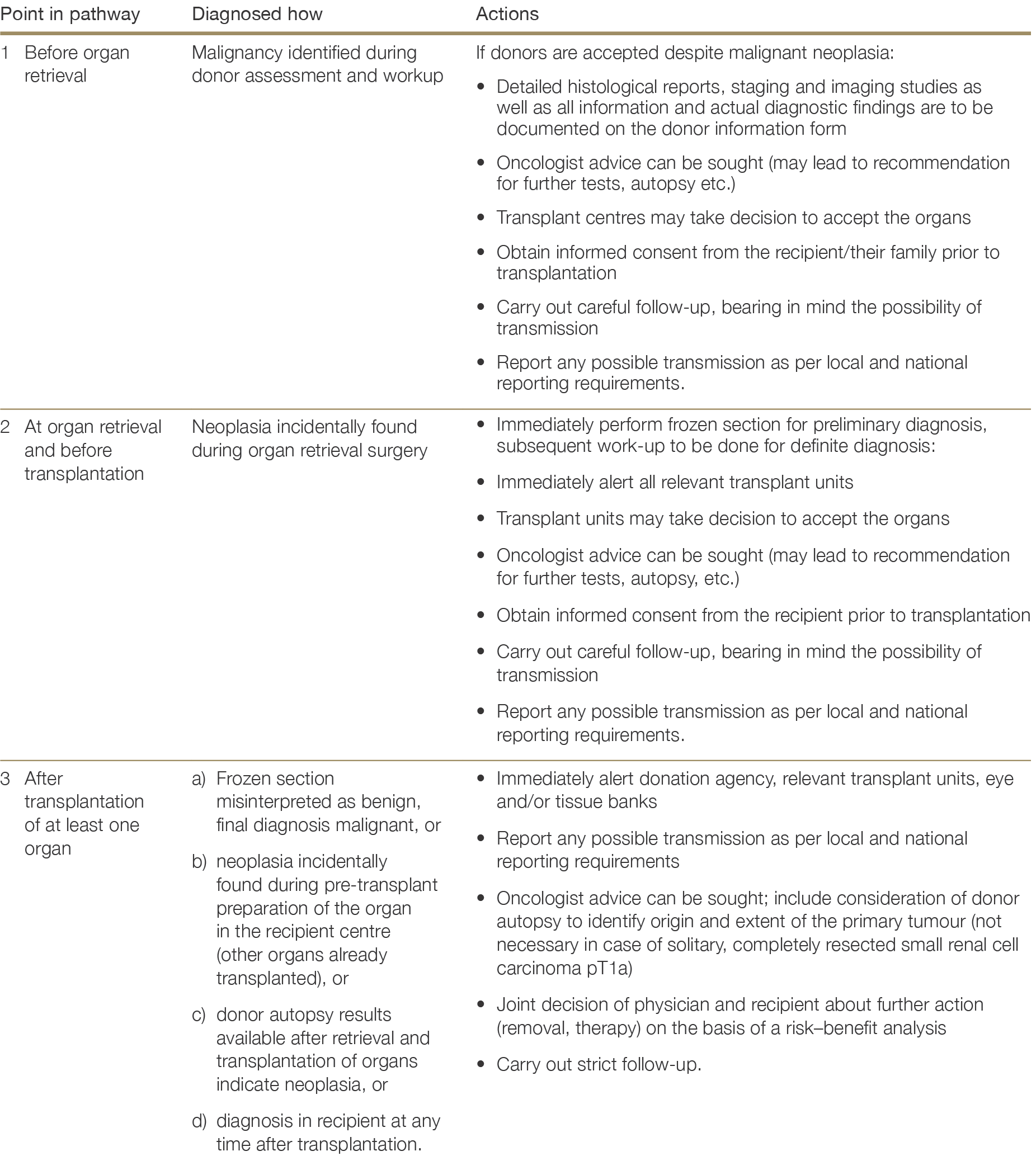
2.5 Risks related to other donor conditions
In addition to the risks of donor-derived infection and malignancy, other pre-existing conditions in the donor may be transmitted via organ donation and transplantation, including some genetic diseases, allergies, and autoimmune diseases. It is critical that any such conditions are thoroughly characterised and conveyed to transplant units as they may influence general donor medical suitability, the suitability for transplantation of specific organs, or require transplant recipients to take particular preventatives measures or receive specific treatments.
2.5.1 Inherited or congenital disorders
There are inherited and genetic diseases which can be transmitted to recipients, depending on the organ transplanted.346 Other genetic diseases may significantly compromise the function of the organ to be transplanted or cause connective tissue disorders, haematopoietic disorders, or predisposition for malignancy. The possibility of an underlying inherited or congenital disorder should be considered in donors with coagulation disturbance (see below), haemochromatosis, mitochondrial deficiency or mental disorder not related to infection, poisoning or malignancy. Presence of an inherited or congenital disorder in the donor must be defined as clearly as possible and communicated to the transplant programs. ALL known gene abnormalities should be communicated and considered in terms of risk of their transmission and degree of organ damage. If the transplant programs are uncertain about how to proceed, then specialist advice should be sought.346 Schielke A, Filomena C, Goumard C et al. Liver transplantation using grafts with rare metabolic disorders. Dig Liver Dis 2015;47:261-70. ×
While it is beyond the scope of these guidelines to consider all potential inherited or congenital diseases that may affect the donation decision, some key examples are considered below.
Ornithine transcarbamylase (OTC) deficiency
OTC deficiency is an example of a latent genetic disorder which may cause cerebral oedema leading to neurological death. The onset of such an event can occur in childhood or later in life and may be precipitated by high protein consumption or unusual exercise. Hyperammonaemia is a key feature and should be measured in any patient with cerebral oedema without a clear cause. Transplantation of the liver from a donor with OTC deficiency carries a high risk of recipient fatality through cerebral oedema and is absolutely contraindicated, though other organs may be safely transplanted.347,348,349347 Ramanthan M, Uppalapu S, Patel NM. Hiding in plain sight: A case of ornithine transcarbamylase deficiency unmasked post liver transplantation. Am J Transplant, 2017; 17: 1405-1408. 348 Caballero F, Ris J, Puig M et al. Successful kidney transplantation from a brain-dead donor with ornithine transcarbamylase deficiency. Transplantation, 2013; 6:e63-e64. 349 Plöchl W, Plöchl E, Pokorny H et al. Multiorgan donation from a donor with unrecognised ornithine transcarbamylase deficiency. Transpl Int, 2001; 14: 196-201. https://www.orpha.net/data/patho/Pro/en/Emergency_Alpha1Antitrypsin-enPro194.pdf ×
Alpha-1-antitrypsin deficiency
Recipients of livers from donors with Alpha-1-antitrypsin deficiency are very likely to develop cirrhosis or fibrosis within months to years of transplantation, necessitating re-transplantation. Organ donation is possible, excluding liver and lung donation in the case of emphysematous patients.350350 https://www.orpha.net/data/patho/Pro/en/Emergency_Alpha1Antitrypsin-enPro194.pdf×
Marfan syndrome and related conditions
Due to the impact of Marfan syndrome on vessel walls and the potential for arterial anastomosis failure, organ donation is generally not recommended from donors with this syndrome.351 Donation of the heart, heart valves, and tissues is absolutely contraindicated, while the utilisation of other organs would require careful consideration. A diagnosis of Marfan syndrome or any other known genetic collagen vascular disorders must be communicated to the transplant programs to allow for careful surgical decision making.351 https://www.orpha.net/data/patho/Pro/en/Emergency_Marfan.pdf ×
2.5.2 Coagulation disorders
Donation of non-liver organs may be considered from donors with inherited coagulation disorders. Liver transplantation is unlikely to be acceptable, although this depends on the nature of the gene defect and the individual risk-benefit assessment given the transplant urgency and likelihood other offers for a particular recipient. In the case of a donor with antithrombin III (ATIII), protein C, protein S or Factor V Leiden deficiency, the defect will be transmitted and the risk of serious thrombotic events in the recipient is increased.352 If liver transplantation is to go ahead, recipients must be willing and able to receive anti-coagulation therapy after transplantation. Any severe form of inherited bleeding disorder such as haemophilia A, B or von Willebrand disease would generally contraindicate liver donation.352 Schuetze S, Linenberge M. Acquired protein S deficiency with multiple thrombotic complications after orthotopic liver transplant. Transplantation, 1999;67:1366-9. ×
Autoimmune-related bleeding or pro-coagulant disorders may also influence suitability of organs for transplantation. Liver transplantation is absolutely contraindicated if high levels of factor VIII inhibitor are detected in the donor prior to organ retrieval.353 A person dying as a result of catastrophic clot arising from anti-phospholipid syndrome would generally be unsuitable due to the risk of thrombosis in donor organs. However, a history of anti-phospholipid syndrome in itself would not contraindicate donation with decision making guided by the severity of the disease.353 Hisatake GM, Chen TW, Renz JF et al. Acquired hemophilia A after liver transplantation. Liver Transpl, 2003;9:523-6. ×
2.5.3 Allergy and anaphylaxis
Where a potential donor has died from anaphylactic allergy to a known allergen, or has a well-known history of serious allergic reactions, this information needs to be communicated to transplant programs and the recipient informed. Passive transfer of type 1 hypersensitivity reaction from donor to recipient has been reported following liver, lung, intestinal, kidney and heart transplantation.354,355,356,357,358,359 Although the exact mechanism of this transfer is unknown, the risk of allergy transfer is higher the more lymphoid material that is transplanted, and is therefore greatest in the case of liver, lung, pancreas and intestinal transplantation.360354 Bradely V, Kemp EH, Dickinson C et al. Vitiligo following a combined liver-kidney transplant. Nephrol Dial Transplant 2009;24:686- 8. Chehade M, Nowak-Wegrzyn A, Kaufmann SS et al. De novo food allergy after intestinal transplantation: a report of three cases. J Pediatr Gastroenterol Nutr 2004;38(5):545-7. 355 Chehade M, Nowak-Wegrzyn A, Kaufmann SS et al. De novo food allergy after intestinal transplantation: a report of three cases. J Pediatr Gastroenterol Nutr 2004;38(5):545-7.356 Legendre C, Caillat-Zucman S, Samuel D et al. Transfer of symptomatic peanut allergy to the recipient of a combined liver-and- kidney transplant. N Engl J Med 1997;337:822-4. 357 Phan TG, Strasser SI, Koorey D et al. Passive transfer of nut allergy after liver transplantation. Arch Intern Med 2003;163:237-9. 358 Khalid I, Zoratti E, Stagner L et al. Transfer of peanut allergy from the donor to a lung transplant recipient. J Heart Lung Transplant 2008;27:1162-4. 359 Boyle RJ, Hardikar W, Tang ML. The development of food allergy after liver transplantation. Liver Transpl, 2005;11:326-30. Legendre C, Caillat-Zucman S, Samuel D et al. Transfer of symptomatic peanut allergy to the recipient of a combined liver and kidney transplant. New Engl J Med, 1997; 337:822-825. ×360 Legendre C, Caillat-Zucman S, Samuel D et al. Transfer of symptomatic peanut allergy to the recipient of a combined liver and kidney transplant. New Engl J Med, 1997; 337:822-825.×
Recipients of organs from donors with a history of allergy and/or anaphylaxis may experience reactions to the same allergens for at least the first 3-6 months post-transplant and must be taught how to avoid such allergen exposure – especially food allergies such as peanut allergy – when the donor history includes anaphylactic reactions.361361 Phan TG, Strasser SI, Koorey D et al. Passive transfer of nut allergy after liver transplantation. Arch Intern Med, 2003; 163(2): 237- 239. ×
2.5.4 Autoimmune disease
Donor history of autoimmune or chronic systemic diseases should be conveyed in detail to the transplant programs, as organ function can be affected and may influence suitability for transplantation. Organs from donors with autoimmune diseases can be used for transplantation after exclusion of significant organ damage and/or infections associated with immunosuppressive treatment of autoimmune disorders.
Certain autoimmune diseases may be transmitted by organ transplantation, such as immune haemolytic anaemia and autoimmune thrombocytopaenia, via the transfer of passenger lymphocytes from the donor to the recipient. This usually occurs in the context of liver or lung transplantation, given the greater number of lymphocytes transferred with these organs, but has also been observed in kidney transplantation.362,363 In many cases this occurs without symptoms, since immunosuppression is also part of the treatment of autoimmune disease. In some cases, however, post-transplant immune-mediated haemolysis can occur, leading to anaemia in the recipient. The potential for immune-mediated haemolysis in the recipient does not preclude organ donation, although the presence of known erythrocyte antibodies in the donor (such as blood group O donor to blood group A or B recipients) indicates prospective monitoring of recipients would be reasonable.362 Friend PJ, McCarthy LJ, Filo RS et al. Transmission of idiopathic (autoimmune) thrombocytopenic purpura by liver transplantation. N Engl J Med 1990;323:807-11. 363 Nadarajah L, Ashmann N, Thuraisinghma R et al. Literature review of passenger lymphocyte following renal transplantation and two case reports. Am J Transplant 2013;13:1594-1600. ×
2.5.5 Poisoning
Poisoning is generally not a contraindication to organ donation – people who have died with, or as a result of, drug toxicity or poisoning can become donors. Depending on the agent, organ function can be affected, which may limit the organs which are suitable for transplantation. The decision about whether to proceed depends on whether the organ being considered is functioning adequately, and assessment should be on an organ-by-organ basis. Most drugs and toxins will have been metabolised and excreted prior to organ donation and therefore residual toxicity to the recipient is not routinely a concern.
In the case of some poisoning agents – pesticides in particular – there may be a risk of delayed organ failure, primarily affecting the liver.364 If the poisoning agent is unusual or if there is uncertainty regarding the impact on organ function, seek toxicology advice.364 Mariage JL, Galliant A, Hantson P. Organ donation following fatal organophosphate poisoning. Transplant Int, 2012; 25:e71-2. Lederman E, W. T., Bavaro M, Arnold J et al. Transfusion-related transmission of yellow fever vaccine virus-California, 2009. Morbidity and Mortality Weekly Report 59(2): 34-37. ×
2.5.6 Donors who have had recent live vaccination
Live vaccines commonly used in Australia are the measles-mumps-rubella (MMR) vaccine, varicella vaccine, zostavax shingles vaccine, and the rotavirus vaccine. Less commonly used are the Bacillus Calmette-Gurin (BCG) tuberculosis vaccine, imojev Japanese encephalitis virus vaccine, yellow fever vaccine, and ACAM2000 mpox vaccine. Of these vaccines, donor transmission to recipients has only been reported for yellow fever vaccine through blood product transfusion, which led to seroconversion in the absence of clinical disease in 3 out of 4 tested recipients (who were all immunocompromised). One recipient died; it is unclear if this death was related to receipt of blood products.365365 Lederman E, W. T., Bavaro M, Arnold J et al. Transfusion-related transmission of yellow fever vaccine virus-California, 2009. Morbidity and Mortality Weekly Report 59(2): 34-37.×
Viremia following vaccination is often studied by nucleic acid detection rather than viral culture.366 Viremia has been found in a small percentage up to 4 weeks post varicella vaccination,367 to 2 weeks post zostavax vaccination,368 to 9 days post measles vaccination of macaque monkeys,369 to 1-2 weeks post yellow fever vaccination,370 and to 8 days after imojev vaccination.371 Disseminated BCG after vaccination is very uncommon and has occurred predominantly in immunocompromised vaccinees.372 Local injection site reactions are common and drainage which remains culture positive can occur up to 6 weeks after vaccination.373 ACAM2000 is not commonly used for mpox vaccination in Australia. This vaccinia virus-based vaccine can be cultured from the inoculation site up to day 42 after vaccination, however, was not detected in blood in one study.374 Rotavirus vaccine is given enterally to infants and remains in the gastrointestinal tract. The Australian Red Cross (Lifeblood) require waiting 4 weeks after live vaccines and 8 weeks after smallpox vaccine before donating blood or platelets.375366 Papp, K. A., B. Haraoui, D. Kumar et al. Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies.” J Cutan Med Surg 2019, 23(1): 50-74. ×367 Gomi, Y., T. Ozaki, N. Nishimura et al. DNA sequence analysis of varicella-zoster virus gene 62 from subclinical infections in healthy children immunized with the Oka varicella vaccine Vaccine, 2008 26(44): 5627-5632. ×368 Levin, M. J., G. Y. Cai et al. Varicella-Zoster Virus DNA in Blood After Administration of Herpes Zoster Vaccine. J Infect Disc 2018, 217(7): 1055-1059. ×369 van Binnendijk, R. S., M. C. Poelen, G. van Amerongen et al. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis, 1997, 175(3): 524-532. Reinhardt, B., R. Jaspert, M. Niedrig et al. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol, 1998, 56(2): 159-167. ×370 Reinhardt, B., R. Jaspert, M. Niedrig et al. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol, 1998, 56(2): 159-167.×371 Monath, T. P., F. Guirakhoo, R. Nichols et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen.” J Infect Dis, 2003, 188(8): 1213-1230. ×372 Talbot, E. A. and R. Frothingham. Meningitis due to Mycobacterium bovis BCG--reactivation or accidental intrathecal inoculation?” Clin Infect Dis, 1996, 23(6): 1335-1336. ×373 Brewer, M. A., K. M. Edwards, P. S. Palmer and H. P. Hinson. Bacille Calmette-Guerin immunization in normal healthy adults.” J Infect Dis, 1994, 170(2): 476-479. ×374 Pittman, P. R., P. M. Garman, S. H. Kim et al. Smallpox vaccine, ACAM2000: Sites and duration of viral shedding and effect of povidone iodine on scarification site shedding and immune response. Vaccine, 2015, 33(26): 2990-2996. Australian Redcross, (2023). “How long after I’ve had a vaccination can I donate?” Retrieved 26 May 2023, 2023, from https://www.lifeblood.com.au/faq/eligibility/medication-and-medical-devices/vaccination. ×375 Australian Redcross, (2023). “How long after I’ve had a vaccination can I donate?” Retrieved 26 May 2023, 2023, from https://www.lifeblood.com.au/faq/eligibility/medication-and-medical-devices/vaccination.×
There is a high degree of uncertainty about the consequences of recent live vaccination of an organ donor for recipients of solid organs. Most live vaccines have not proven to be viscerotropic and viremia rates reduce over several weeks. Prophylactic measures to recipients may protect against clinical manifestations.
The consequences of live vaccine virus transmission may be ameliorated by antivirals or immunotherapies. Varicella zoster immunoglobulin (VZIG) and antivirals or recipient immunity could reduce the effects of varicella and zostavax vaccines. Normal human immunoglobulin (NHIG) or recipient immunity could reduce the effects of MMR vaccine. Mycobacterial agents (isoniazid, rifampicin, ethambutol, moxifloxacin, levofloxacin) are active against BCG vaccine.
Recommendation
Caution should be applied when considering solid organ transplantation from donors who have had live vaccination in the last 4 weeks. It is recommended to discuss risk and mitigation with an infectious diseases physician. Duration since donor vaccination (>2 weeks likely to have lower risk of transmission) and possible risk mitigation are factors to consider. Administration of VZIG and/or antivirals should be considered for organ recipients (particularly who are not immune to VZV) of a donor vaccinated recently with varicella vaccine or zostavax. NHIG should be considered for organ recipients (particularly who are not immune to measles, mumps or rubella) of a donor recently vaccinated with MMR. Anti–mycobacterial agents may be considered for organ recipients of a donor recently vaccinated with BCG.
2.6 Organ distribution and allocation
The allocation of organs is a complex process, influenced by a number of factors including medical need, medical urgency, recipient capacity to benefit, donor/recipient matching, and logistical factors.
The allocation process and specific allocation criteria vary depending on the type of organ to be transplanted, as outlined in Chapters 4 to 10 of this document. Clinical decisions about organ allocation can be very difficult due to the number and variability of factors that must be considered. The development of organ matching algorithms within OrganMatch has streamlined the offering and allocation of donor organs across Australia. Clinical input to assess the medical need and recipient capacity to benefit must be at the forefront of every decision, and it is for this reason that every attempt should be made to uphold the principles of allocation embodied in the Ethical Guidelines.376376 National Health and Medical Research Council (2025). Ethical guidelines for cell, tissue and organ donation and transplantation in Australia. Canberra: National Health and Medical Research Council. ×
Transplant units should use donated organs in a way that balances medical need with the likelihood of successful transplantation, taking into account the following general criteria when considering potential recipients for organs:
Length of time waiting for a transplant, taken from the time that the illness progressed to a point that a transplant would be of immediate benefit
Important medical factors, such as the closeness of tissue-matching and matching of organ quality to estimated recipient survival
The urgency of the transplant given the likely deterioration of the patient’s health without transplantation, especially if patient survival is immediately threatened by that deterioration
Medical need, in terms of how sick the patient is without transplantation, and the prospects for transplantation to improve the patient’s outcome (both in terms of survival and quality of life)
Logistical considerations in making the transplant available to the recipient within an appropriate timeframe (see below)
Anthropomorphic measurements for some organs, especially hearts and lungs.
The time between removal of the organ from the donor and its implantation into the recipient (the ischaemic time) is critical to post-transplantation outcomes. State based allocation (organs are allocated within their home state), assists in minimising this ischaemic time but is not always most appropriate. Further information on National Allocation of solid organs can be found in the TSANZ-OTA National Standard Operating Procedure, Organ Allocation, Organ Rotation, Urgent Listing.
Organs donated in New Zealand may be offered to Australian units, and vice versa where logistics and ischaemic times allow. If there is no suitable recipient in the donor country or as required by an urgent listing.
2.7 Vigilance and Surveillance
The Australian Vigilance and Surveillance System (AVSS) for Organ Donation for Transplantation is the central location and system for reporting SAERs ( reported serious adverse events or reactions) relating to organs from deceased donors and is managed by the Organ and Tissue Authority (OTA).
The AVSS undertakes work in the following areas:
a. work in parallel with state and territory clinical incident management systems in deceased organ donation and transplantation
b. receive and coordinate responses to SAER notifications
c. monitor, record and retrospectively analyse SAER notifications
d. inform future processes in organ donation for transplantation, and
e. improve the safety and quality of organ donation and transplantation, thereby improving patient outcomes.
The National Vigilance and Surveillance system works in parallel with the existing jurisdictional clinical incident management systems. The clinical management and investigation of serious adverse events and reactions (SAERs) remains the responsibility of the hospital and jurisdictions in which the incident occurs. States and territories continue to be responsible for local reporting processes including communication with associated clinicians and patients, investigation of the incident; and the response to an incident including feedback, local policy and clinical practice review. While cases of donor derived disease transmission are rare, the immediate reporting and investigation of any post-transplant infection in the recipient and the notification of the donation agency and other recipients from the same donor is imperative to prevent/minimise harm to those exposed.
A key component of the Organ and Tissue Authority (OTA) National Vigilance and Surveillance System is the Vigilance and Surveillance Expert Advisory Committee (VSEAC). The purpose of VSEAC is to monitor the performance of the vigilance and surveillance system and advise OTA on emerging risks identified in the organ and tissue donation and transplantation sectors and provide recommendations for action where appropriate.
Further information can be obtained by emailing [email protected].